Association Analysis of TEC Polymorphisms with Aspirin-Exacerbated Respiratory Disease in a Korean Population
Article information
Abstract
The tyrosine-protein kinase Tec (TEC) is a member of non-receptor tyrosine kinases and has critical roles in cell signaling transmission, calcium mobilization, gene expression, and transformation. TEC is also involved in various immune responses, such as mast cell activation. Therefore, we hypothesized that TEC polymorphisms might be involved in aspirin-exacerbated respiratory disease (AERD) pathogenesis. We genotyped 38 TEC single nucleotide polymorphisms in a total of 592 subjects, which comprised 163 AERD cases and 429 aspirin-tolerant asthma controls. Logistic regression analysis was performed to examine the associations between TEC polymorphisms and the risk of AERD in a Korean population. The results revealed that TEC polymorphisms and major haplotypes were not associated with the risk of AERD. In another regression analysis for the fall rate of forced expiratory volume in 1 second (FEV1) by aspirin provocation, two variations (rs7664091 and rs12500534) and one haplotype (TEC_BL2_ht4) showed nominal associations with FEV1 decline (p = 0.03-0.04). However, the association signals were not retained after performing corrections for multiple testing. Despite TEC playing an important role in immune responses, the results from the present study suggest that TEC polymorphisms do not affect AERD susceptibility. Findings from the present study might contribute to the genetic etiology of AERD pathogenesis.
Introduction
Asthma is a disease that is caused by inflammation in the lung and bronchus and is affected by genetic and environmental influences. Aspirin-exacerbated respiratory disease (AERD), which was first reported in 1922, is a type of asthma. AERD is characterized by the following three symptoms: bronchial asthma, aspirin sensitivity, and nasal polyposis [1, 2, 3]. It has been reported that 10-20% of asthma patients have aspirin sensitivity, whereas 1-2% of the non-asthma population shows aspirin sensitivity [4, 5]. Although the mechanisms of AERD pathogenesis are still not fully understood, inflammatory responses by overproduction of leukotrienes are regarded as the main pathogenesis of AERD.
Aspirin is a commonly used medication, which belongs to the non-steroidal anti-inflammatory drugs. Despite being a widely used medication, aspirin intake also causes various side effects, including manifested gastrointestinal ulcer, stomach bleeding, and tinnitus, especially in higher doses. Although the side effects of aspirin are not common, these effects have been reported in about 10% of adult asthmatics. In AERD pathogenesis, aspirin inhibits the activation of cyclooxygenase-1 enzyme, leading to block of production of prostaglandin and thromboxane. This mechanism causes overproduction of leukotrienes, such as leukotriene B4, leukotriene C4, leukotriene D4, and leukotriene E4 [6, 7].
The tyrosine-protein kinase Tec (TEC) is a member of the non-receptor tyrosine kinases and has critical roles in cell signaling transmission, calcium mobilization, gene expression, and transformation [8]. It has been known that TEC family kinases are associated with various intracellular signaling mechanisms, such as cytokine receptors and lymphocyte surface antigens [9, 10]. In particular, it was demonstrated that the TEC family proteins are involved in regulation of leukotriene secretion via the mast cell signaling pathway [11]. Therefore, we hypothesized that TEC polymorphisms might be involved in AERD pathogenesis. In the present study, 38 TEC single nucleotide polymorphisms (SNPs) were genotyped in a total of 592 subjects, which comprised 163 AERD cases and 429 aspirin-tolerant asthma (ATA) controls, to examine the associations between TEC polymorphisms and AERD susceptibility.
Methods
Study subjects
Subjects in this study were recruited from the Asthma Genome Research Center, comprising the hospitals of Soonchunhyang, Chunnam, Chungbuk, Seoul National, and Chung-Ang Universities in Korea. All subjects provided written informed consents, and the study protocols were approved by the institutional review board of each hospital. Diagnosis of AERD was performed according to a modified method as previously described [12]. We also performed aspirin challenge in subjects with a history of aspirin hypersensitivity, presence of urticaria, nasal polyp, and sinusitis. The AERD case group included patients with 20% or greater decreases in forced expiratory volume in 1 second (FEV1) or 15% to 19% decreases in FEV1 with naso-ocular or cutaneous reactions, whereas subjects showing a rate of FEV1 decline less than 15% without extrabronchial nasal or skin symptoms were included in the ATA group. The clinical diagnostic factors for the present study are summarized in Table 1.
SNP selection and genotyping
To investigate the associations between TEC polymorphisms and the risk of AERD, we selected candidate SNPs based on allele frequencies in the Asian population, linkage disequilibrium (LD) status, and National Center for Biotechnology information. The data for selection were obtained from the International HapMap database (http://hapmap.ncbi.nlm.nih.gov/). Genotyping of 38 TEC polymorphisms was performed in a total of 592 subjects, including 163 AERD cases and 429 ATA controls. Genotyping was carried out with 20 ng of genomic DNA by TaqMan assay using ABI prism 7900HT sequence detection system software version 2.3 (Applied Biosystems, Foster City, CA, USA) in all subjects. Assay IDs of all SNPs used in TaqMan assay are listed in Supplementary Table 1.
Statistics
We applied a widely used measure of linkage disequilibrium to all pairs of biallelic loci: Lewontin's D' (|D'|) [13] and r2. Haplotypes of each individual were inferred using the PHASE algorithm (ver. 2.0), developed by Stephens et al. [14]. Linear regression analysis was performed to examine the differences in the rates of decline in FEV1 following aspirin challenge among the genotypes and major haplotypes. The data were managed and analyzed using Statistical Analysis System, version 9.2 (SAS Inc., Cary, NC, USA). Associations for AERD under the logistic model were adjusted by smoking status, atopy, body mass index (BMI), age, and sex (male = 0, female = 1). Significant associations are shown in boldface (p < 0.05).
Results and Discussion
We recruited a total of 592 subjects, which consisted of 163 AERD cases and 429 ATA controls, for the present study. According to the results, four clinical characteristics showed significant differences between the case and control groups (Table 1). The fall rate by aspirin provocation was significantly higher in AERD subjects than ATA controls (24.63 ± 16.11 vs. 3.54 ± 4.85; p = 0.0001). Percentage of predicted FEV1 in the AERD subjects showed decreased lung function than in ATA subjects (87.58 ± 16.94 vs. 91.66 ± 16.87; p = 0.009). Also, age of first medical examination and BMI were lower in AERD cases than ATA controls. The other diagnostic factors showed no significant differences between the case and control group.
In the present study, 38 TEC polymorphisms and 12 major haplotypes were used for the association analyses. Locations of the polymorphisms are shown in a genetic map of TEC with their LD status (Fig. 1A). Minor allele frequencies (MAFs) of the SNPs in Korean subjects are displayed in Supplementary Table 2 with their allele change, position, and p-value for Hardy-Weinberg equilibrium. All SNPs were in Hardy-Weinberg equilibrium. The LD blocks were obtained by using HaploView software, and the haplotypes of each LD block were calculated by PHASE software (Fig. 1B and 1C). We used major haplotypes that had frequencies higher than 0.05 for further analyses. The minor haplotypes that had frequencies lower than 0.05 were merged and presented as 'Others.' To compare genetic differences among ethnicities, we obtained MAFs of Caucasians, Han Chinese, Japanese, and Africans from the NCBI database (dbSNP) (Supplementary Table 2) and calculated LD blocks using genotype data from the International HapMap database (Supplementary Fig. 1). As a result, Asian populations showed similar MAFs, whereas the other populations showed distinct differences. However, we did not find any correlation in LD status in any ethnicity.

Schematic physical map, haplotypes and linkage disequilibrium (LD) plot of TEC. (A) Polymorphisms identified in TEC. Coding exons are marked by shaded blocks and untranslated region by white blocks. The LD coefficients (r2) are based on the genotypes of Korean samples. (B) Haplotypes are calculated using genotypes of TEC polymorphisms in a Korean population. Only those with frequencies over 0.05 are shown in tables. Haplotypes with frequencies lower than 0.05 are merged into "Others." (C) LD coefficients (|D'|) among the selected SNPs based on the genotypes of whole study subjects in this study.
Logistic analyses were performed to investigate the associations between TEC polymorphisms and the risk of AERD. The result from analyses revealed that all SNPs and major haplotypes were not associated with the risk of AERD (Table 2). On the other hand, two SNPs (rs7664091, p = 0.04 and rs12500534, p = 0.03) and one major haplotype in LD block 2 (TEC_BL2_ht4, p = 0.03) showed marginal associations in the regression analysis with the decline rate of FEV1 (Supplementary Table 3). However, the association signals of these polymorphisms disappeared after performing corrections for multiple testing (data not shown). Taken together, these results indicate that the TEC polymorphisms are not associated with AERD susceptibility, at least in the Korean population. The lack of associations in this study suggests that although TEC plays an important role in immune responses, TEC variants do not directly affect decreased pulmonary function by aspirin uptake. However, considering the fact that the difference in frequency of polymorphisms showed different effects in various populations, replication studies in AERD subjects in other ethnicities are recommended.
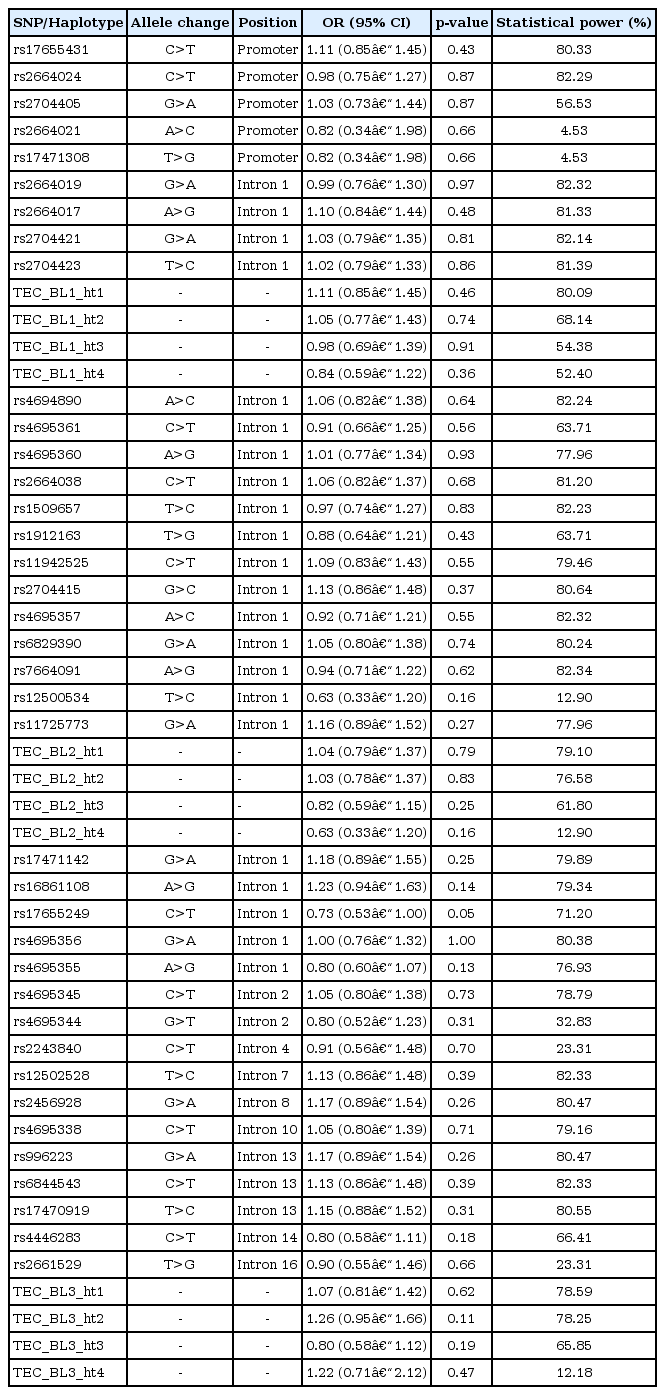
Association analysis of TEC polymorphisms and haplotypes with the risk of AERD in a Korean population
The present study still had several limitations. First, an average statistical power of 66.41% indicated an insufficient sample size. However, the rare condition of AERD made it difficult to recruit subjects in the Korean asthma cohort. Second, SNPs in coding sequence were not selected, due to very low frequencies, although non-synonymous SNPs (nsSNP) in exonic regions could affect risk of the disease. Further studies are required to examine the molecular role of nsSNPs in TEC.
In conclusion, we hypothesized that TEC might impact on AERD susceptibility. However, our results showed that 38 TEC polymorphisms and 12 major haplotypes were not associated with the risk of AERD. Although further studies are required to investigate the exact role of the TEC SNPs in immune responses, the preliminary results of the present study may provide useful information for AERD pathogenesis.
Acknowledgments
This work was supported by a grant from the Korea Health 21 R&D Project (A010249). This work was supported by a grant from the Priority Research Centers Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2012-0006690). The DNA samples were generously provided by Soonchunhyang University, Bucheon Hospital Biobank, and a member of the National Biobank of Korea, supported by the Ministry of Health, Welfare and Family Affairs, Republic of Korea.
References
Supplementary materials
Supplementary data including three tables and one figure can be found with this article online at http://www.genominfo.org/src/sm/gni-12-58-s001.pdf.
Supplementary Table 1
Assay IDs of 38 single nucleotide polymorphisms in TEC gene
Supplementary Table 2
Allele distribution of TEC polymorphisms
Supplementary Table 3
Regression analysis of TEC polymorphisms and haplotypes with the fall rate of FEV1 by aspirin provocation in a Korean population
Supplementary Fig. 1
Linkage disequilibrium (LD) plots for TEC gene in five ethnicities. LD plots were calculated by using data from International HapMap Project. (A) LD of Korean. (B) LD of Han Chinese. (C) LD of Japanese. (D) LD of Caucasian. (E) LD of African.
