|
 |
- Search
| Genomics Inform > Volume 20(1); 2022 > Article |
|
Abstract
2-Methoxy-1,4-naphthoquinone (MNQ) has been shown to cause cytotoxic towards various cancer cell lines. This study is designed to investigate the regulatory effect of MNQ on the key cancer genes in mitogen-activated protein kinase, phosphoinositide 3-kinase, and nuclear factor кB signaling pathways. The expression levels of the genes were compared at different time point using polymerase chain reaction arrays and Ingenuity Pathway Analysis was performed to identify gene networks that are most significant to key cancer genes. A total of 43 differentially expressed genes were identified with 21 up-regulated and 22 down-regulated genes. Up-regulated genes were involved in apoptosis, cell cycle and act as tumor suppressor while down-regulated genes were involved in anti-apoptosis, angiogenesis, cell cycle and act as transcription factor as well as proto-oncogenes. MNQ exhibited multiple regulatory effects on the cancer key genes that targeting at cell proliferation, cell differentiation, cell transformation, apoptosis, reduce inflammatory responses, inhibits angiogenesis and metastasis.
The process of carcinogenesis is relay on over-expression of cancer genes through gene amplification, inappropriate expression of normal genes, or mutations in genes [1,2]. Accumulation of genetic mutations and epigenetic alterations are needed to induce a normal cell to a transformed cell [3] and eventually lead to cancer formation. Cancer is mainly about the active and “gain-of-function” process of the oncogenes with loss of function of tumor suppressor genes [4]. The acquisition of oncogenes and tumor suppressor genes was shown at different times during the tumor progression in different type of tumors [5].
The complication of carcinogenesis involves several cancer hallmarks. These underlying principles are describing how normal cells transform to malignant or tumor cells. Hallmarks of cancer refers to behaviors of cancer cells that sustained to proliferative signals, resisted to cell death signals, evading from growth suppressors, enabling replicative immortality, genome instability and mutation, continues tumor-promoting inflammation, deregulating cellular energetic, avoiding immune destruction, inducing angiogenesis, and activating invasion and metastasis [5]. Multiple signaling pathways regulate each aforementioned cancer hallmark.
Cancer cells are known to have alterations in multiple cellular signaling pathways involving the affected genes [6]. Aberrant cancer signaling pathways always have been characterized with their complexities regarding the alterations and deregulations of the genes involved [7]. These multiple dysfunctional signaling pathways implicated in cancer development have become one of the factors that lead to the complexity of carcinogenesis and difficulties faced in cancer research. With the understanding of cancer complexities in the link between these signaling pathways, the search for curing cancer can possibly done by disrupting these altered signaling pathways [8]. Non-toxic natural products can be used to disrupt these altered signaling pathways; these natural products can activate cell death signals in pre-cancerous or cancerous cells without affecting the normal cells [9]. Besides, these natural products can be used alone or in combination with chemotherapeutic drugs.
More than 10 cancer signaling pathways have been identified [10]. Studies on the aberrant signaling pathways are not necessarily limited to only one or two specific signaling pathways towards cancer progression. In many cases, a few of the pathways would be studied altogether to clearly know the effects of the complexities and the interactions between these signaling networks towards carcinogenesis. In term of inhibiting signal transduction, suppression of multiple signaling pathways is better than suppression on single pathway at a time [11]. In a feedback loop theory, suppression of one signaling pathway would activate another pathway automatically. This is a phenomenon where cancer cells prolong the survival as mutated cell [12]. In all, better understanding and accurate dissection of all these signaling pathways are important; this is because they are vital for elucidating the most appropriate and reliable target molecules for the design of effective cancer therapy [13]. For instance, many potential chemo preventive drugs have been tested on multiple dysfunctional proteins that are involved in multiple dysfunctional cancer signaling pathways.
Impatiens balsamina, Linn from the family of Balsaminaceae, is an ornamental plant that has been used to treat various skin diseases locally. 2-Methoxy-1,4-naphthoquinone (MNQ), isolated from pericarps of Impatiens balsamina Linn. has been shown to cause cytotoxic towards various cancer cell lines [14], show anti-tumor-promoting activity in HepG2 and Raji cells [15,16], trigger apoptotic pathway and the upper stream modulator of many cancer pathways [17], inhibit protein kinase C expressions in Raji cells [16], and suppress the invasion and migration of MDA-MB-231 [18]. Furthermore, MNQ also has been shown to inhibit WNT signaling in STF/293 cells [19], altered proteins related to cytoskeletal functions and regulations, mRNA processing, protein modifications, and oxidative stress responses [20].
PKC is one of the protein kinases that play a role in carcinogenesis and maintenance of malignant phenotype. Moreover, it is also the core upper stream kinase involved in mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K), and nuclear factor кB (NF-κB) pathways that regulate many down-stream proteins implicated in these cancer signaling pathways. In our previous study, MNQ has been demonstrated to inhibit PKC βI, δ, and ζ [16]; therefore, this study was continued to reveal the genes that are possibly regulated by MNQ in MAPK, PI3K, and NF-κB pathways.
Phorbol 12-myristate 13-acetate (PMA) and sodium n-butyrate (SnB) were obtained from Sigma. MNQ was isolated from the pericarps of Impatiens balsamina, Linn. The compound was prepared in dimethyl sulfoxide and stored at 4ºC. The reagents obtained from Qiagen (Hilden, Germany) were RT2 First Strand Kit (containing GE buffer, 5× BC3 buffer, RE3 reverse transcriptase mix, P2 control, and RNase-free water), RT2 STBR Green ROX FAST mastermix (containing HotStart DNA Taq Polymerase, PCR buffer, dNTP mix [dATP, dCTP, dGTP, dTTP], SYBR Green dye ROX mastermix).
Raji cells obtained from Riken Cell Bank, Japan were maintained in commercial Roswell Park Memorial Institute Media (RPMI-1640) supplemented with 10% fetal bovine serum (FBS) (Thermo Fisher, Waltham, MA, USA). The cells were incubated in a humidified atmosphere at 37°C with 5% CO2 incubator (ESCO, Hatboro, PA, USA).
The high-performance liquid chromatography (HPLC) chromatogram of the isolated MNQ was first identified by comparing the HPLC chromatogram of MNQ HPLC chromatogram obtained by Teng [14] in our previous work. After that, mass spectrometry spectrum was compared to NIST mass spectral data. The identified MNQ was then isolated and subjected to HPLC spiking analysis and gas chromatography and mass spectrometry spiking analysis. Isolated MNQ structure was confirmed with nuclear magnetic resonance (NMR). Proton and carbon NMR chemical shift results were compared to Teng [14].
Raji cells (5 × 105 cells/mL) were incubated in 1 mL of RPMI 1640 medium (supplemented with 10 % FBS) containing 0.05 µM PMA, 3 mM SnB, and MNQ in a 24-well plate and then incubated at 37°C for 6, 12, 24, and 48 h in a CO2 incubator. PMA and SnB was used as inducer and enhancer respectively to cause Epstein-Barr virus activation in Raji cells and directly transform Raji cells into promotion stage.
RNA of the treated Raji cells was extracted according to the manufacturer’s instruction using RNeasy Mini Kit (Qiagen). RT2 RNA QC PCR Array was used to confirm the purity of RNA and excluded substandard samples prior to RT2 Profiler PCR Arrays analysis. The RNA samples extracted were at high-quality RNA, in which the A260:A230 ratio obtained was greater than 2.0 and the A260:A280 ratio attained was in between 1.8 to 2.0. Next, the cDNA was synthesized using the RT2 First Strand Kit (Qiagen) following the manufacturer’s instructions. The genomic DNA elimination mix was prepared accordingly to the manual given by Qiagen.
The cDNA was added with RT2 SYBR Green ROX FAST Mastermix according to manufacturer’s protocol. RT2 Profiler PCR Array for the detection of key cancer genes regulation in MAPK, PI3K, and NF-κB pathways were tested in three independent experiments. The genes expression results were calculated using results generated by the RT cycler and the ∆∆CT method. The CT values for all wells were exported to PCR Array Data Analysis Template Excel sheet (downloaded at http://www.sabiosciences.com/dataanalysis.php) and uploaded to web-based software at www.sabiosciences.com/pcrarraydataanalysis.php. This software helps in calculating CT values collected and shows p-values and fold change for each gene. The connection between genes was analyzed using web-based resources Gene Network Central Pro at http://gncpro.sabiosciences.com/gncpro/gncpro.php.
The identified key cancer genes were first uploaded onto Qiagen’s Ingenuity Pathway Analysis (IPA) system for core analysis and then overlaid with the global molecular network in the ingenuity pathway knowledge base. IPA was performed to identify canonical pathways, diseases and functions, and gene networks that are most significant to key cancer genes and to categorize differentially expressed genes in specific molecular and cellular functions. Thresholds of two-fold or greater in changes in expression and a p-value of 0.05 or less for significance were used to filter the findings from the analysis with IPA software.
A total of 43 statistically significant expressed genes were identified, which was 19.72 % of the total of 218 cancer key genes studied. All the 43 genes were statistically significant expressed in Raji cells after treated with 0.05 μM PMA, 3 mM SnB, and 40 μM MNQ (p < 0.05, fold change > 4.0). The fold changes of all statistically significant genes involved in these pathways are shown in Fig. 1 (MAPK pathway), Fig. 2 (PI3K pathway), and Fig. 3 (NF-κB pathway). While Fig. 4 shows bar chart for fold change of all statistically significant genes involved in more than one pathway. Negative and positive values denoted down-regulation and up-regulation of gene expression, respectively.
From the 43 genes identified, MNQ up-regulated 21 genes and down-regulated 22 genes. The identified 21 up-regulated genes were apoptotic genes (BAD, EGR1, FOXO1, FOXO3, ITGB1, LTA, TNF, TNFRSF1A, TNFRSF10B, TRADD, YWHAH), tumor suppressor genes (APC, RB1, and TP53) and cell cycle regulation genes (CDKN1A, CDKN1B, CDKN2A, CDKN2D, MTOR, LAMTOR3, and PTEN). The confirmed 22 down-regulated genes were anti-apoptotic genes (HSPB1, NFKB1, NFKB2, NFKB1A, NFKB1E, RPS6KA1), angiogenesis genes (IL6, TIMP1), cell cycle regulation genes (ARAF, AKT2, AKT3, BRAF, EGFR, HRAS, IGF1, IGF1R, KRAS, NRAS, PDGFRA), transcription factors (FOS and JUN), proto-oncogenes (BCL3).
Among all the 43 statistically significant expressed genes, 12 genes (ARAF, BRAF, CDKN1A, CDKN1B, CDKN2A, CDKN2D, EGFR, KRAS, LAMTOR3, NRAS, RB1, and TP53) are involved in MAPK pathway (Table 1), 14 genes (AKT2, AKT3, APC, BAD, FOXO1, FOXO3, IGF1, IGF1R, ITGB1, MTOR, PDGFRA, PTEN, RPS6KA1, and YWHAH) are involved in PI3K pathway (Table 2), 10 genes (BCL3, IL6, LTA, NFKB2, NFKB1E, TIMP1, TNF, TNFRSF1A, TNFRSF10B, and TRADD) are involved in NF-κB pathway (Table 3), and seven genes (EGR1, FOS, HRAS, HSPB1, JUN, NFKB1, and NFKB1A) are involved in all three pathways (Table 4).
Overall, the functions of 21 up-regulated and 22 down-regulated genes regulated by MNQ are listed in Tables 5 and 6, respectively.
The IPA software system enables systemic analysis of 43 statistically significant genes in a biologic context. Network of up/down-regulated genes were then algorithmically generated based on their inter-relationships. Five major networks were identified and included with functions related to cell death and survival, cellular development, cell cycle, proliferation and cell to cell signaling (Table 7). Canonical pathways were then identified and analyzed from the IPA libraries that were most significant to our common gene data set. Top statistically significant canonical pathways included PTEN, glioblastoma multiforme, PI3K/AKT, glioma signaling 1 and molecular mechanisms of cancer signaling pathways are shown in Table 8 (p < 0.05) and network contains differentially expressed 43 genes are shown in Fig. 5.
The common cancer pathways are MAPK, PI3K, NF-κB, STAT, NOTCH, TP53, RB1, WNT, and Hedgehog pathways. These pathways play crucial roles in survival and maintain tumourigenesis properties of cancer cells [21]. All these pathways are complement to each other forming a very complicated network, which has not been fully studied yet. In term of inhibiting signal transduction, suppression of multiple signaling pathways is better than suppression on single pathway at a time [11]. In a feedback loop theory, suppression of one signaling pathway would activate another pathway automatically. This is a phenomenon where cancer cells can prolong the survival as mutated cell [12]. Thus, this study focuses to determine the modulatory effects of MNQ on the key genes involved in MAPK, PI3K, and NF-κB pathways.
Overall, MNQ significantly regulated 43 genes (19.72%) from the total 218 cancer key genes studied in these three signaling pathways (Figs. 1‒3). These 43 genes that consist of 21 up-regulated genes (Table 5) and 22 down-regulated genes (Table 6) have different functions in regulating tumorigenesis in cancer cells (Table 7). The regulatory effects of these 43 genes in various cellular processes are discussed in following sections.
In this study, MNQ activated the apoptosis, tumor suppressor and cell cycle regulatory activities in MAPK pathway, where the genes of LAMTOR3, TP53, RB1, CDKN1A, CDKN1B, CDKN2A and CDKN2D and were up-regulated across all time points (Table 1, Fig. 1). MNQ induced the expression of P53 and RB1 transcription factors to exert its suppressive effect in preventing the progression of cell cycle in Raji cells. Upon the treatment of MNQ, MNQ could possibly cause DNA damage [22] and thus induce the expression of P53. In respond to DNA damage, P53 induces cyclin-dependent kinase inhibitors such as CDKN1A, CDKN1B, CDKN2A, CDKN2D that responsible for cell cycle arrest. The expression of late endosomal/lysosomal adaptor, MAPK and MTOR activator 3 (LAMTOR3) in MNQ-treated Raji could possibly explain that Raji cells tried to sustain their survival in the presence of the abovementioned genes in inducing cell cycle arrest and apoptosis. This phenomenon can be observed from Fig. 1 where the LAMTOR3 expression were gradually increased in amount from 6 to 48 h treatment. In addition, EGRF, epidermal growth factor receptor involved in cell cycle was down-regulated by MNQ. Furthermore, proto-oncogene genes that are involved in cell growth and differentiation such as ARAF, BRAF, KRAS, HRAS and NRAS were also down-regulated across all time points. Overall, MNQ mainly caused cell cycle arrest, supressed cell proliferation and induced apoptosis in Raji cells via MAPK pathway.
In this study, MNQ activated tumor suppressor, apoptosis and cell cycle regulation through upregulating PTEN, FOXO1, FOXO3, YWHAH, MTOR, ITGB1, BAD, and APC genes in PI3K pathway (Table 2, Fig. 2), respectively. Among these up-regulated genes, adenomatous polyposis coli (APC) was found to be the most highest expressed gene across all time points. APC is a tumor suppressor that indirectly regulate a number of key genes involved in cell proliferation. Besides, it also plays a role in cell migration [23]. PTEN is another tumour suppressor gene up-regulated by MNQ that generally involved in the regulation of cell cycle, and it is known to be the target of many anticancer drugs. FOXO1 and FOXO3 have been reported to trigger apoptosis via regulating genes that responsible for cell death such as BIM and PUMA [24]. BAD is a known pro-apoptotic gene that initiate apoptosis. YWHAH encodes for 14-3-3 protein eta has been reported to regulate a wide variety of signaling pathways, and mainly mediated these signaling pathways by binding to phosphoserine-containing proteins. MTOR that encode for mTOR complex 1 and 2 proteins has been reported to regulate cell growth, cell proliferation, cell motility and cell survival [25]. ITGB1 gene encodes Integrin beta-1 surface receptor that are involved in cell proliferation, cell adhesion and recognition as well as metastatic diffusion of tumor cells [26]. In our previous study [27], MNQ was demonstrated to induce apoptosis in A549 lung adenocarcinoma cell through oxidation triggered JNK and p38 MAPK signaling pathways. In this study, the abovementioned listed gene were up-regulated throughout all time points of treatment, this could explain Raji cells strived to survive upon the treatment of MNQ that creating the oxidative stress environment. Raji cells strived to survive via the abovementioned genes that are mainly involved in cell growth, proliferation, and survival. On the contrary, MNQ down-regulated PDGFRA, AKT2, AKT3, RPS6KA1, IGF1, and IGF1R, genes. PDGFRA gene has been reported to instruct the making of platelet-derived growth factor receptor alpha (PDGFRA). PDGFRA proteins activate signaling pathways to control many important cellular processes such as cell growth, division and survival. Mutation on PDGFRA has been reported in gastrointestinal stromal tumors and in chronic myeloid leukemia. Besides, highly phosphorylated PDGFRA has been observed in non‒small cell lung cancer and rhabdomyosarcoma [22], AKT2 and AKT3 are the serine/threonine kinases that regulate cellular metabolism, cell proliferation, cell survival, cell growth and angiogenesis. These genes are highly activated in many cancer cells. The AKT protein kinase has been indicated to transduce growth factors to oncogenes and target the proteins to induce tumor development. Thus, inhibition of the AKT kinase is workable in cancer prevention as down-regulation of AKT signaling is a strategy to prevent cancer formation [28]. Akt kinases has been known to inhibit FOXOs transcriptional functions and contribute to cell survival, cell growth and cell proliferation. RPS6KA1 encodes ribosomal protein S6 kinase alpha-1 kinase that phosphorylate various substrates of the MAPK singling pathway, which is implicated in controlling cell growth and differentiation. RPS6KA1 is also known as RSK1 that present in cytoplasm. RSK1 is an ERK effector that is involved mainly in nuclear signaling, cell growth, survival, and cell proliferation. It has been reported to regulate SRF, c-Fos, and Nur77 transcription factors. RSK1 stimulated cell growth by regulating c-Fos and promotes cyclin D1 expression. Besides, RSK1 has been demonstrated to inhibit neuronal NO synthase in response to mitogenic signaling. RSK1 is highly expressed in prostate and breast cancers. RSK1 triggered survival signals via the Ras/ERK signaling pathway and protected cancer cells from apoptosis. RSK1 is also involved in cell cycle regulation by phosphorylating CDKN1B and preventing CDKN1B translocation into nucleus and thus inhibited G1 progression. RSK1 also promoted cell survival by suppressing the functions of pro-apoptotic proteins BAD and DAPK1. MNQ down-regulated insulin-like growth factor 1 (IGF1) and insulin-like growth factor 1 receptor (IGF1R) significantly. IGF family is a humoral mediator of growth hormone (GH) [29]. The IGF signaling pathway that control endocrine system and regulate cell growth and development has been reported to have a pathogenic role in cancer [30]. IGF has been reported to increase cancer cells growth and cells resistance to chemotherapy and radiation by decreasing functions of targeted agents in GH independent manner [31]. IGF1R is highly expressed and biologically active in small cell lung cancer, pediatric high-grade gliomas, and Ewing’s Sarcoma [32].
For NF-κB pathway, MNQ was found to up-regulate TRADD, LTA, TNFRSF1A, TNFRSF10B, and TNF genes, and down-regulate NFKB2, NFKB1E, IL6, TIMP1, and BCL3 genes. LTA is a cytokine produced by lymphocytes that belong to tumor necrosis factor family. LTA bound to TNFRSF1A/TNFR1, TNFRSF1B/TNFBR, TNFRSF14 and induced apoptosis. TNFRSF1A is expressed in transformed cells to trigger apoptosis or inflammation [33]. Mutations in TNFRSF1A has been studied to display resistance towards apoptosis [34]. TNFRSF1A signals apoptosis through caspase-8 [35]. Tumor necrosis factor-α (TNFα) has been indicated to promote apoptosis in human endothelial cells through TNFRSF1A and trigger caspase-2 and p53 activation. It took part in variety cellular responses, such as survival, differentiation, proliferation, apoptosis, and migration [33]. TNFα is a pro-inflammatory cytokine regulated by TNFRSF1A and TNFRSF1B [36]. TNFRSF1A has been shown to promote the recruitment of TNFR-associated factors (TRAFs), FAS-associated via death domains (FADDs), and TNFR-associated via death domains (TRADDs). TNFRs interact with TRAFs, FADDs and TRADDS to control apoptosis. TNFα has been reported to bind with TNFRSF1A to induce apoptosis by activating caspase-8 [37]. Tumor necrosis factor receptor superfamily, member 10b (TNFRSF10B) is a member of the TNF-receptor superfamily, it has the similar function with TNFRSF1A. TNFRSF10B has been shown to interact with FADD [38], caspase 10, and caspase 8 and induced apoptosis in cancer cells. TRADD contains a death domain that interact with TNFRSF1A or TNFR1 to activate apoptosis. It has been reported to bind with TRAF2 to reduce the inhibitor-of-apoptosis proteins expression, and thus suppressed TRAF2-mediated apoptosis. TRADD activated apoptosis through FADD and caspase-8 activation [39]. STAT1, FADD, TNFRSF1A, and TNFRSF25 are the proteins interact with TRADD. NFKB1, NFKB2, NFKB1A, and NFKB1E are the genes encode for protein members of NF-κB family. Two angiogenesis genes of IL6 and TIMP1 were down-regulated by MNQ. IL6 is an inflammatory cytokine that modulate growth and differentiation in tumor cells through STAT3 signaling pathway. Overexpression of IL6 has been reported to be associated with tumor progression through inhibition of cancer cell apoptosis, stimulation of angiogenesis, and drug resistance. Clinical studies have been reported IL6 protein as a regulator that associate with many types of cancers such as multiple myeloma, non‒small cell lung adenocarcinoma, prostate cancer, colorectal cancer, renal cell carcinoma, breast cancer, and ovarian cancer [40,41]. Tissue inhibitor of metalloproteinases 1 (TIMP1) has become a marker of prognosis and indicator for checking the clinical response in cancer treatment because TIMP1 is elevated in breast, colon, and prostate cancer patient plasma [42,43]. BCL3 was the only down-regulated proto-oncogene by MNQ. BCL3 is a proto-oncogene deregulated in solid tumors. BCL3 has been revealed to induce proliferation and inhibit apoptosis. BCL3 activated STAT3, an aggressive oncogene in human cancer and promoted metastasis [44,45]. In leukemia cells, BCL3 has been demonstrated to up-regulate myc genes and lead to the formation of aggressive B-cell leukemia. Other report mentioned that NF-κB activated BCL3 to act as transcriptional co-activator. BCL3 contains two transactivating domain and can form homodimers with NF-κB1 (p50) or NF-κB2 (p52). It has been reported that BCL3 was up-regulated by cytokines such as TNFα, interleukin (IL)-1, IL-4, IL-6, IL-10, IL-12, and adiponectin [46-54]. These cytokines were activated by AP1 [46] and STAT3 [45,55]. In addition, BCL3 was down-regulated by p53 [56].
In a nutshell, MNQ up-regulated apoptotic genes (BAD, EGR1, FOXO1, FOXO3, ITGB1, LTA, TNF, TNFRSF1A, TNFRSF10B, TRADD, and YWHAH), tumor suppressor genes (APC, RB1, and TP53), and cell cycle genes (CDKN1A, CDKN1B, CDKN2A, CDKN2D, MTOR, LAMTOR3, and PTEN). On the contrary, MNQ down-regulated anti-apoptotic genes (HSPB1, NFKB1, NFKB2, NFKB1A, NFKB1E, and RPS6KA1), angiogenesis genes (IL6 and TIMP1), cell cycle genes (ARAF, AKT2, AKT3, BRAF, EGFR, HRAS, IGF1, IGF1R, KRAS, NRAS, and PDGFRA), transcription factors (FOS and JUN), and proto-oncogenes (BCL3).
The findings of this study have revealed the capability of MNQ in regulating the key cancer genes in MAPK, PI3K, and NF-κB signaling pathways. The regulatory effects exerted by MNQ are targeting at cell proliferation, cell differentiation, cell transformation, induce apoptosis, reduce inflammatory responses, inhibits angiogenesis and metastasis. These results underline the need to further investigate the mechanism of actions of MNQ at each molecular level of these genes.
Notes
Acknowledgments
This work was supported by Fundamental Research Grant Scheme (FRGS) Grant (FRGS/1/11/SKK/UTAR/03/5) from Ministry of Higher Education (MOHE), Malaysia.
Fig. 1.
Fold change of statistically significant genes regulated by MNQ in MAPK pathway. All experiments were performed individually with triplicate. p < 0.05 and fold change > 4.0 was set as the significant threshold. MNQ, 2-methoxy-1,4-naphthoquinone; MAPK, mitogen-activated protein kinase.
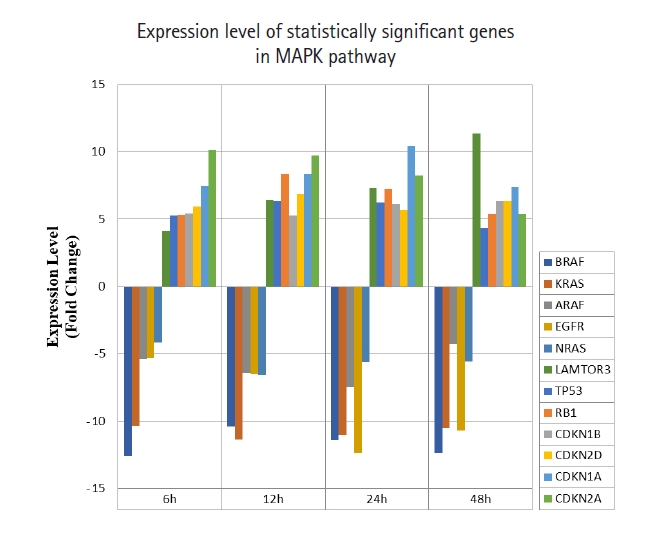
Fig. 2.
Fold change of statistically significant genes regulated by MNQ in PI3K pathway. All experiments were performed individually with triplicate. p < 0.05 and fold change > 4.0 was set as the significant threshold. MNQ, 2-methoxy-1,4-naphthoquinone; PI3K, phosphoinositide 3-kinase.
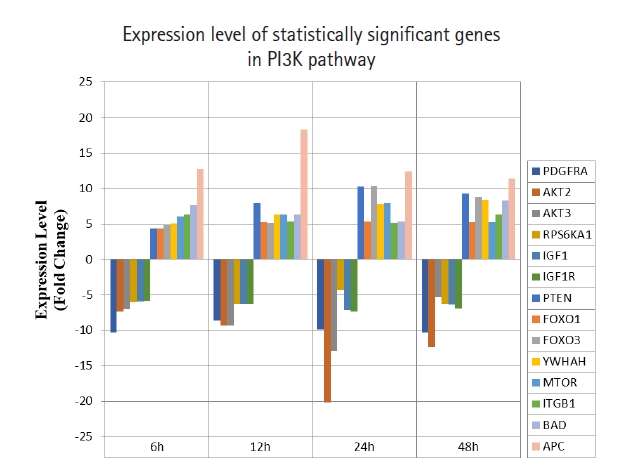
Fig. 3.
Fold change of statistically significant genes regulated by MNQ in NF-κB pathway. All experiments were performed individually with triplicate. p < 0.05 and fold change > 4.0 was set as the significant threshold. MNQ, 2-methoxy-1,4-naphthoquinone; NF-κB, nuclear factor кB.
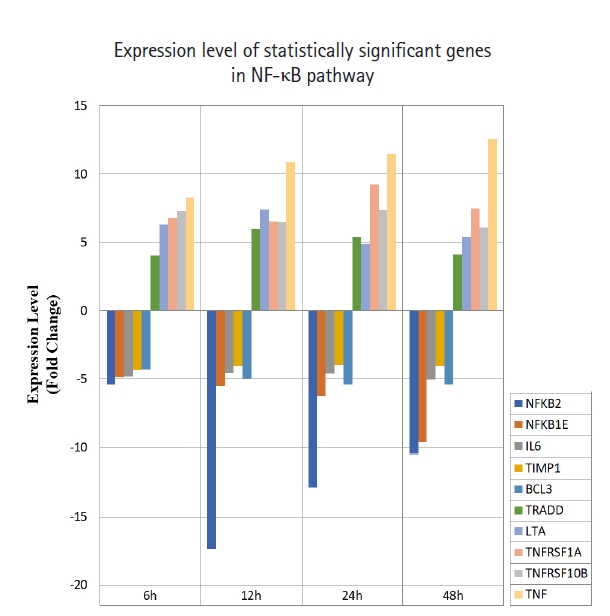
Fig. 4.
Fold change of statistically significant genes regulated by MNQ. These genes are involved in more than a pathway. All experiments were performed individually with triplicate. p < 0.05 and fold change > 4.0 was set as the significant threshold. MNQ, 2-methoxy-1,4-naphthoquinone.
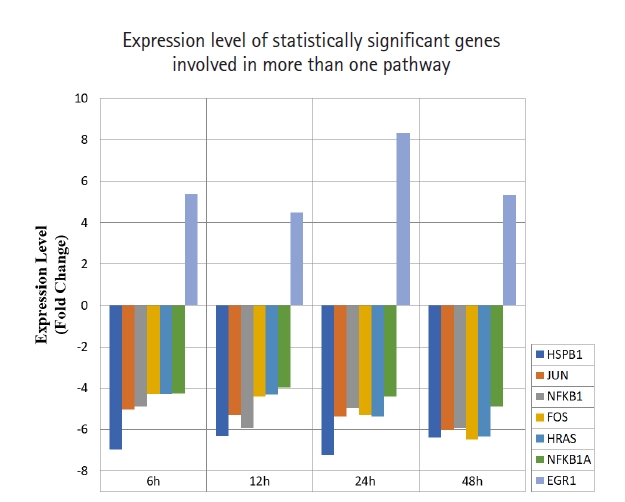
Fig. 5.
Network contains differentially expressed 43 genes. Top functions of the genes were related to molecular and cellular functions and physiological system development and function. Ingenuity Pathway Analysis network legend is on the left side.

Table 1.
Up-regulated and down-regulated genes in MAPK pathway
Table 2.
Up-regulated and down-regulated genes in PI3K pathway
Table 3.
Up-regulated and down-regulated genes in NF-κB pathway
Table 4.
Down-regulated genes in MAPK, PI3K, or NF-κB pathways
Table 5.
Functions of 21 up-regulated genes by MNQ
Table 6.
Functions of 22 down-regulated genes by MNQ
Table 7.
Molecular and cellular functions
Table 8.
List of the genes in most significantly up/down-regulated top five canonical pathways
References
1. Rak J, Yu JL. Oncogenes and tumor angiogenesis: the question of vascular "supply" and vascular "demand". Semin Cancer Biol 2004;14:93–104.

3. Gordon K, Clouaire T, Bao XX, Kemp SE, Xenophontos M, de Las Heras JI, et al. Immortality, but not oncogenic transformation, of primary human cells leads to epigenetic reprogramming of DNA methylation and gene expression. Nucleic Acids Res 2014;42:3529–3541.


4. Wang LH, Wu CF, Rajasekaran N, Shin YK. Loss of tumor suppressor gene function in human cancer: an overview. Cell Physiol Biochem 2018;51:2647–2693.


6. Laubenbacher R, Hower V, Jarrah A, Torti SV, Shulaev V, Mendes P, et al. A systems biology view of cancer. Biochim Biophys Acta 2009;1796:129–139.



7. Pelullo M, Zema S, Nardozza F, Checquolo S, Screpanti I, Bellavia D. Wnt, Notch, and TGF-beta pathways impinge on Hedgehog signaling complexity: an open window on cancer. Front Genet 2019;10:711.


9. Khazaei S, Esa NM, Ramachandran V, Hamid RA, Pandurangan AK, Etemad A, et al. In vitro antiproliferative and apoptosis inducing effect of Allium atroviolaceum Bulb extract on breast, cervical, and liver cancer cells. Front Pharmacol 2017;8:5.



10. Schulz WA. Molecular Biology of Human Cancers: An Advanced Student’s Textbook. Dordrecht: Springer, 2007. pp. 113–144.
11. Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: 'liking', 'wanting', and learning. Curr Opin Pharmacol 2009;9:65–73.



12. Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 2009;9:550–562.


13. Bell HS, Ryan KM. Intracellular signalling and cancer: complex pathways lead to multiple targets. Eur J Cancer 2005;41:206–215.


14. Teng O. Isolation and characterisation of a cytotoxic napthoquinone from Impatiens balsamina. M.S. thesis Kampar: Universiti Tunku Abdul Rahman, 2010.
15. Ding ZS, Jiang FS, Chen NP, Lv GY, Zhu CG. Isolation and identification of an anti-tumor component from leaves of Impatiens balsamina. Molecules 2008;13:220–229.



16. Yew WT, Kitson L, Hock AH, Chiew GS, Mooi LY. 2-Methoxy-1,4-naphthoquinone (MNQ) suppresses protein kinase C βI, δ, and ζ expression in Raji cells. J Appl Pharm Sci 2015;5:001–005.
17. Tan SY. Differential protein expression in K562 following exposure to MNQ isolated from Impatiens balsamina, Linn. M.S. thesis Kampar: Universiti Tunku Abdul Rahman, 2011.
18. Liew K, Yong PV, Lim YM, Navaratnam V, Ho AS. 2-Methoxy-1,4-Naphthoquinone (MNQ) suppresses the invasion and migration of a human metastatic breast cancer cell line (MDA-MB-231). Toxicol In Vitro 2014;28:335–339.


19. Mori N, Toume K, Arai MA, Koyano T, Kowithayakorn T, Ishibashi M. 2-methoxy-1,4-naphthoquinone isolated from Impatiens balsamina in a screening program for activity to inhibit Wnt signaling. J Nat Med 2011;65:234–236.


20. Liew K, Yong PV, Navaratnam V, Lim YM, Ho AS. Differential proteomic analysis on the effects of 2-methoxy-1,4-naphthoquinone towards MDA-MB-231 cell line. Phytomedicine 2015;22:517–527.


21. Nakanishi M, Shimada M, Niida H. Genetic instability in cancer cells by impaired cell cycle checkpoints. Cancer Sci 2006;97:984–989.


22. McDermott U, Ames RY, Iafrate AJ, Maheswaran S, Stubbs H, Greninger P, et al. Ligand-dependent platelet-derived growth factor receptor (PDGFR)-alpha activation sensitizes rare lung cancer and sarcoma cells to PDGFR kinase inhibitors. Cancer Res 2009;69:3937–3946.



24. Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta 2011;1813:1978–1986.


25. Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov 2009;8:627–644.



26. Song J, Zhang J, Wang J, Cao Z, Wang J, Guo X, et al. beta1 integrin modulates tumor growth and apoptosis of human colorectal cancer. Oncol Rep 2014;32:302–308.


27. Ong JY, Yong PV, Lim YM, Ho AS. 2-Methoxy-1,4-naphthoquinone (MNQ) induces apoptosis of A549 lung adenocarcinoma cells via oxidation-triggered JNK and p38 MAPK signaling pathways. Life Sci 2015;135:158–164.


29. Daughaday WH, Hall K, Raben MS, Salmon WD Jr, van den Brande JL, van Wyk JJ. Somatomedin: proposed designation for sulphation factor. Nature 1972;235:107.


30. Gallagher EJ, LeRoith D. Diabetes, cancer, and metformin: connections of metabolism and cell proliferation. Ann N Y Acad Sci 2011;1243:54–68.


31. Arnaldez FI, Helman LJ. Targeting the insulin growth factor receptor 1. Hematol Oncol Clin North Am 2012;26:527–542.



32. McKinsey EL, Parrish JK, Irwin AE, Niemeyer BF, Kern HB, Birks DK, et al. A novel oncogenic mechanism in Ewing sarcoma involving IGF pathway targeting by EWS/Fli1-regulated microRNAs. Oncogene 2011;30:4910–4920.



33. Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 2003;3:745–756.


34. D'Osualdo A, Ferlito F, Prigione I, Obici L, Meini A, Zulian F, et al. Neutrophils from patients with TNFRSF1A mutations display resistance to tumor necrosis factor-induced apoptosis: pathogenetic and clinical implications. Arthritis Rheum 2006;54:998–1008.


35. Lee RU, Saland S, Sullivan S. Tumor necrosis factor receptor-associated periodic syndrome as a cause of recurrent abdominal pain in identical twins and description of a novel mutation of the TNFRSF1A gene. J Pediatr Gastroenterol Nutr 2013;56:e22–23.


36. Miura A, Honma R, Togashi T, Yanagisawa Y, Ito E, Imai J, et al. Differential responses of normal human coronary artery endothelial cells against multiple cytokines comparatively assessed by gene expression profiles. FEBS Lett 2006;580:6871–6879.


37. MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal 2002;14:477–492.


38. Gajate C, Mollinedo F. Cytoskeleton-mediated death receptor and ligand concentration in lipid rafts forms apoptosis-promoting clusters in cancer chemotherapy. J Biol Chem 2005;280:11641–11647.


39. Bender LM, Morgan MJ, Thomas LR, Liu ZG, Thorburn A. The adaptor protein TRADD activates distinct mechanisms of apoptosis from the nucleus and the cytoplasm. Cell Death Differ 2005;12:473–481.


40. Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest 2007;117:3846–3856.



41. Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev 2012;38:904–910.


42. Gong Y, Scott E, Lu R, Xu Y, Oh WK, Yu Q. TIMP-1 promotes accumulation of cancer associated fibroblasts and cancer progression. PLoS One 2013;8:e77366.



43. Wurtz SO, Schrohl AS, Mouridsen H, Brunner N. TIMP-1 as a tumor marker in breast cancer: an update. Acta Oncol 2008;47:580–590.


44. Wakefield A, Soukupova J, Montagne A, Ranger J, French R, Muller WJ, et al. Bcl3 selectively promotes metastasis of ERBB2-driven mammary tumors. Cancer Res 2013;73:745–755.


45. Maldonado V, Espinosa M, Pruefer F, Patino N, Ceballos-Canciono G, Urzua U, et al. Gene regulation by BCL3 in a cervical cancer cell line. Folia Biol (Praha) 2010;56:183–193.

46. Rebollo A, Dumoutier L, Renauld JC, Zaballos A, Ayllon V, Martinez AC. Bcl-3 expression promotes cell survival following interleukin-4 deprivation and is controlled by AP1 and AP1-like transcription factors. Mol Cell Biol 2000;20:3407–3416.



47. Heissmeyer V, Krappmann D, Wulczyn FG, Scheidereit C. NF-kappaB p105 is a target of IkappaB kinases and controls signal induction of Bcl-3-p50 complexes. EMBO J 1999;18:4766–4778.



48. Elliott SF, Coon CI, Hays E, Stadheim TA, Vincenti MP. Bcl-3 is an interleukin-1-responsive gene in chondrocytes and synovial fibroblasts that activates transcription of the matrix metalloproteinase 1 gene. Arthritis Rheum 2002;46:3230–3239.


49. Kuwata H, Watanabe Y, Miyoshi H, Yamamoto M, Kaisho T, Takeda K, et al. IL-10-inducible Bcl-3 negatively regulates LPS-induced TNF-alpha production in macrophages. Blood 2003;102:4123–4129.


50. Hu X, Nesic-Taylor O, Qiu J, Rea HC, Fabian R, Rassin DK, et al. Activation of nuclear factor-kappaB signaling pathway by interleukin-1 after hypoxia/ischemia in neonatal rat hippocampus and cortex. J Neurochem 2005;93:26–37.


51. Valenzuela JO, Hammerbeck CD, Mescher MF. Cutting edge: Bcl-3 up-regulation by signal 3 cytokine (IL-12) prolongs survival of antigen-activated CD8 T cells. J Immunol 2005;174:600–604.


52. Brocke-Heidrich K, Ge B, Cvijic H, Pfeifer G, Loffler D, Henze C, et al. BCL3 is induced by IL-6 via Stat3 binding to intronic enhancer HS4 and represses its own transcription. Oncogene 2006;25:7297–7304.


53. Vatsveen TK, Ro TB, Hella H, et al. High expression of BCL3 in human myeloma cells is associated with increased proliferation and inferior prognosis. Eur J Haematol 2009;82:354–363.



54. Folco EJ, Rocha VZ, Lopez-Ilasaca M, Libby P. Adiponectin inhibits pro-inflammatory signaling in human macrophages independent of interleukin-10. J Biol Chem 2009;284:25569–25575.



- TOOLS
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 4,730 View
- 136 Download
- Related articles in GNI