 |
 |
- Search
| Genomics Inform > Volume 20(2); 2022 > Article |
|
Abstract
According to long-term projections, by 2030, the worldâs population is predicted to reach 7.5 billion individuals, and there will be roughly 27 million new cancer cases diagnosed. The global burden of breast cancer (BC) is expected to rise. According to the Ministry of Health-Iraqi Cancer Registry, cancer is the second largest cause of death after cardiovascular disease. This study investigated the interleukin-18 (IL18) single-nucleotide polymorphisms (SNPs) â607C/A rs1946518 and â137G/C rs187238 using the sequence-specific amplification-polymerase chain reaction approach. Regarding the position â607C/A, there was a highly significant difference between the observed and expected frequencies in patients and controls (Ď2 = 3.16 and Ď2 = 16.5), respectively. The AA and CA genotypes were associated with significantly increased BC risk (odds ratio [OR], 3.68; p = 0.004 and OR, 2.83; p = 0.04, respectively). Women with the A allele had a 5.03-fold increased susceptibility to BC. The C allele may be a protective allele against BC (OR, 0.19). Although position â137G/C showed no significant differences in the CC genotype distribution (p = 0.18), the frequency of the CC genotype was significantly higher in patients than in controls. In contrast, patients had a significantly higher frequency of GC genotypes than controls (p = 0.04), which was associated with an increased risk of developing BC (OR, 2.63). The G allele frequency was significantly lower in patients than in controls (55.0% vs. 76.2%, respectively). This SNP may be considered a common genotype in the Iraqi population, with the wild-type G allele having a protective function (OR, 0.19) and the mutant C allele having an environmental effect (OR, 2.63).
Breast cancer (BC) is the most common cancer in females worldwide, with nearly 2.3 million new cases diagnosed in 2020. It accounts for approximately 11.7% of all cancer cases and 24.5% of all cancer cases in females [1]. Since 2008, the global incidence of BC has increased by more than 20%, and the death rate has risen by 14% [2]. As a result, research on relevant tumor indicators for early diagnosis and monitoring is required, and recent studies have concentrated on the role of the immune system in cancer progression [3].
BC is a multi-step process that involves numerous genetic changes, such as oncogene activation and cancer suppressor gene inactivation [4]. The gene encoding interleukin (IL)-18 is found on chromosome 11 at positions 11q22.2âq22.3 and has six exons in humans. IL-18 is a multidirectional cytokine that regulates the immune response in various ways; in 1989, IL-18 was first described as an âinterferon-inducing factor.â IL-18 plays an essential role in stimulating natural killer cells, and cellular anticancer activities also enhance the expansion of Th1 and cell activation. Furthermore, IL-18 increases the expression of adhesion-related molecules, nitric oxide synthase enzyme synthesis, and chemokine production [5]. IL-18, in combination with IL-2, causes a Th2 cell response and the production of IL-4 and IL-13. Simultaneously, IL-18 can reduce antitumor immunity in a programmed-death-1 (PD-1) dependent manner. PD-1 is a co-inhibitory receptor that constitutes one of the top checkpoints. Many polymorphisms in the IL-18 promoter region affect transcript factor binding locations [6], which could be IL-18 expression quantitative trait loci. Several studies have found that cytokine gene polymorphisms impact cytokine production, which may be linked to disease [7].
Single-nucleotide polymorphisms (SNPs) are found in the IL18 gene, particularly in the promoter region bound to â607G/T (rs1946518) and â137G/C (rs187238). These SNPs (â137G/C and â607G/T) in the IL18 gene promoter region are expected to affect IL-18 expression and activity [8]. Arimitsu et al. [9] found that monocytes in individuals with the 137G/G genotype produced considerably more IL-18 than those in individuals with the 137G/C genotype. Furthermore, a link between these two SNPs and cancer susceptibility has been established [10]. Research on the relevance of IL18 polymorphisms in BC risk remains contentious. To our knowledge, only three studies have examined the influence of IL18 genetic variations (â137G/C and â607A/C) on BC susceptibility; Khalili-Azad et al. [11] studied the effect of IL18 polymorphisms (â607A/C and â137G/C) on BC risk in 200 patients and 206 healthy controls. They discovered that CC homozygosity for the â137G/C polymorphism lowered the risk of BC [11]. Other studies found that the IL18 â607A/C polymorphism contributed to an increased risk of BC [12,13]. In light of this information, it is necessary to investigate IL18 SNPs in Iraqi women with BC.
After the ethical committee of the Medical City directorate approved this study, 100 women who visited the oncology teaching hospital's breast clinic were recruited from January 28, 2020 to August 11, 2022. Group 1 included 60 women who had recently been histopathologically diagnosed with BC and provided written informed consent for participation in the study. Group 2 included 40 healthy women who served as controls. Patients with other systemic diseases and those taking any hormone-modifying drug were excluded from the study.
Each patient and control had 8-10 mL of blood taken. Blood samples were taken from the cubital vein and placed directly into an anticoagulant tube containing EDTA. Genomic DNA extraction was performed using a Norgen Biotek kit (Thorold, ON, Canada). The optical density ratio at 260/280 nm was used to assess the quantity and quality of isolated DNA Using a Nano-Drop device (Qubit 4, Invitrogen, Waltham, MA, USA). It was preferable to have a balance of 1.7â1.9. Electrophoresis on a 1% agarose gel was performed to corroborate the findings. Until the genotyping analysis, the DNA samples were stored at â20°C.
Polymorphisms were investigated by utilizing sequence-specific amplification-polymerase chain reaction (SSP-PCR) at locations â607C/A rs1946518 and â137G/C rs187238 within the promoter region of IL18. For the targeted location â607C/A, a common reverse primer and two specific forward primers were utilized, with an amplified product size of 196 bp, and a forward control primer was used to amplify a 301-bp fragment covering the polymorphism region as an internal control (Table 1).
In the location â137G/C, a common reverse primer and two specific forward primers were also utilized, with an amplified product size of 261 bp, and a forward control primer was used to amplify a 446-bp fragment covering the polymorphism region as an internal control (Table 2). The polymerase chain reaction (PCR) reactions were carried out in a volume of 25 ÎźL, including 12.5 ÎźL of 2Ă Go-Taq Green Master mix (Promega, Madison, WI, USA), 3 ÎźL of genomic DNA, and 6.5 ÎźL of nuclease-free water. All reaction mixtures contained one sequence-specific primer, one common reverse primer, and one internal position control primer, each at a concentration of 1 ÎźL. As a result, each piece of DNA was subjected to two PCR tests: one for the F1 wild-type allele and one for the F2 mutant allele [8]. The assays were carried out in a thermocycler (Applied Biosystems, Waltham, MA, USA). Denaturation was carried out at 95°C for 5 min, followed by 30 cycles at 94°C for 30 s, 57°C for 30 s, 72°C for 40 s, and 72°C for 10 min. Then, the PCR results were visualized using electrophoresis on 2% agarose gel, and the molecular weight was calculated using a 100-bp DNA ladder.
The genotype of cytokines was statistically analyzed using the SPSS version 25 (IBM Corp., Armonk, NY, USA). The allele frequencies of the cytokine genes were calculated by the direct gene counting method. A freely available online calculator was used to calculate whether there was a significant departure from Hardy-Weinberg (H-W) equilibrium for two alleles (http://www.had2know.com/academics/Hardy-Weinberg equilibrium-calculator-3-alleles.html). H-W equilibrium is the expected frequency of genotypes if mating is non-assortative and there are no mutations from one allele to another. When there are two alleles for a particular gene, A and B, and their respective population frequencies are p and q, the expected frequencies of the genotypes AA, AB, and BB are p2, 2pq, and q2, respectively. The Pearson chi-square test was used to assess whether there were significant differences between the observed and expected frequencies. The alleles and genotypes of cytokines were presented as percentages and frequencies, and the two-tailed Fisher exact test was calculated to assess the significance of differences between their distributions in BC patients and controls. Odds ratios (ORs) were also estimated to define the association between cytokine alleles and genotypes with BC. OR values can range from between 0 and 1 (for a negative association) to more than 1 (for a positive association).
This study analyzed the distribution of the genotype and allele frequencies of the rs1946518 polymorphism (at the â607C/A position) in patients and controls (Fig. 1). This polymorphism presented three genotypes (CC, CA, and AA) that corresponded to two alleles (T and A) in BC patients and controls. The genotype frequencies in both groups were not in agreement with H-W equilibrium, and there were highly significant differences between the observed and expected frequencies in both patients and controls (Ď2 = 3.16 and Ď2 = 16.5), respectively (Table 3).
The CC genotype was significantly less common in patients than in controls (15.0% vs. 65.5%, p < 0.001), and the magnitude of this negative association was 0.11. However, the AA genotype frequency was higher in patients (51.7%) than in controls (22.5%); this difference was significant (OR, 2.83; p = 0.004). The CA genotype frequency was also significantly higher in patients than in controls (33.3% vs. 15.0%; OR, 3.68; p = 0.04). The C allele frequency was lower in patients than in controls (25.0% vs. 38.7%), while the A allele frequency was higher (75.0% vs. 61.3%, respectively). A negative association was found for the C allele (OR, 0.19) and a positive association for the A allele (OR, 5.03) (p < 0.001 for both) (Table 4).
The rs187238 genetic polymorphism of the IL18 gene was investigated at position â137G/C (Fig. 2), and presented with three genotypes (GG, GC, and CC) that corresponded to two alleles (G and C). The results of patients were in agreement with the expected H-W equilibrium. However, in the control group, the results were not in agreement with the expected (H-W) equilibrium, and there was a highly significant difference between the observed and expected frequencies (Ď2 = 5.74) (Table 5).
At the position â137G/C in IL18, patients had a significantly higher frequency of the GC genotype than controls (43.3% vs. 22.5%; OR, 2.63; p = 0.04). In contrast, the frequency of the CC genotype was significantly higher in patients than in controls (23.3% and 12.5%; OR, 2.13). Therefore, the G allele frequency was significantly lower in patients than in controls (55.0% vs. 76.2%, respectively), with a highly significant difference (p = 0.002). A negative association was found for the G allele (OR, 0.38) and a positive association for the C allele (OR, 2.36) (Table 6).
The present study investigated the two most commonly studied SNPs of the IL18 gene (â607C/A and â137G/C). The patients and controls both showed deviation from H-W equilibrium for the -607C/A genotype, which may have been related to BC or intermarriage in Arab Iraqi society between relatives. This result aligns with that of a previous study on BC [14].
Although the distribution of the â137G/C genotype in the patient group was consistent with H-W equilibrium, this was not the case for the control group, which showed a highly significant difference between the observed and expected frequencies (Ď2 = 5.74). This SNP may be considered a common genotype in the Iraqi population, with the wild-type G allele having a protective function, reducing susceptibility, and the mutant C allele having an environmental effect.
These SNPs' genotypes and alleles showed significant differences between BC patients and controls. The present study observed that the IL18 â607 CA and AA genotypes were present in about 85% of BC cases. The wild-type CC genotype had a low frequency (about 15%) in BC patients, which may highlight the role of the IL18 â607 polymorphism in the pathogenesis of disease. The A allele was an environmental effect allele, while the C allele had a preventive fraction because the CC genotype showed the highest frequency in the control group (70%). These highly significant findings for the AA and CA genotypes at position IL18 â607 suggest that this polymorphism may play a role in cancer progression.
Several studies on IL18 polymorphisms have been conducted in various populations in multiple countries; one of them has found a link between IL18 polymorphisms and the risk of BC [15]. The IL18 â607C/A polymorphism may be linked to an increased risk of BC in Asian and mixed populations [16]. Furthermore, the present results showed that the IL18 rs1946518 SNP might play a role in BC because the wild-type allele C of the â607 SNP had a protective effect against BC (OR, 0.19). In contrast, the mutant allele A had a positive association (OR, 5.03) suggesting an etiological impact; therefore, women who carry allele A of â607 may be more susceptible to BC than women who have allele C. However, the results of some studies were mixed; in a study involving 72 BC patients and 93 control women, Fathi Maroufi et al. [17] discovered that the IL18 -607A/C polymorphism was not linked to BC in an Iranian population sample. The IL18 rs187238 polymorphism results showed no significant differences according to the CC genotype frequencies between BC patients and controls. Nonetheless, it was interesting to note in the present study that the heterozygous GC genotype had an OR of 2.63 for the patient group, implying that the mutant allele C may have had an environmental effect on the Iraqi population, conferring susceptibility to BC, while the wild-type allele G had a preventive effect against BC (OR, 0.38). Our findings show that the IL18 -137G/C polymorphism is associated with the development of BC. The conversion of G (guanine) to C (cytosine) at position -137G/C of the IL18 gene removes a nuclear factor binding site for histone-4 transcriptional factor-1 [18]. Genetic variants have been considered the most critical cancer risk factors. Although high-penetrant capability genes (e.g., BRCA1 and BRCA2) have strong links to BC, low-penetrant susceptibility genes that predispose individuals to the disease have yet to be identified; nonetheless, immune responses and surveillance may be affected by genetic variability in a sequence of immune regulatory genes [19]. The IL18 promoter polymorphism â137G/C has previously been linked to various cancers in different populations, including esophageal squamous cell malignant tumors, prostate cancer in the Chinese population [20], colorectal cancer in Greek people [21], and ovarian cancer in native Hawaiians [22]. Additional case-control studies on BC and gastric cancer progression have been published [23,24]. However, there is no link between type 2 diabetes mellitus development and the IL18 â137G/C gene polymorphism [25].
Notes
Acknowledgments
The authors are grateful to everyone who volunteered for this study. We want to express our heartfelt gratitude to the entire Institute of Science at Cankiri Karatekin University staff for their kind assistance. Also, many thanks go to all the staff at the Oncology Teaching Hospital, Medical City, Baghdad, especially the Cancer Research Laboratory, for their help with the diagnosis and sample collection.
Fig. 1.
Agarose gel electrophoresis image that shows the sequence-specific amplification-polymerase chain reaction product analysis of interleukin-18 (IL18) â607 (rs1946518) (C/A) gene polymorphism. Where M, marker (100â1,200 bp), the presence of C or A allele were observed at 196-bp product size. The (CC) wild type homozygote were showed in C allele only, the (AA) mutant type homozygote were showed in A allele only, whereas the (C/A) heterozygote were showed in both C and A allele, internal control at 301-bp product size.

Fig. 2.
Agarose gel electrophoresis image that shows the sequence-specific amplification-polymerase chain reaction product analysis of interleukin-18 (IL18) â137 (rs187238) (G/C) gene polymorphism. Where M, marker (100â1,200 bp), The presence of G or C allele were observed at 261-bp product size. the (GG) wild type homozygote were showed in G allele only, the (CC) mutant type homozygote were showed in C allele only, whereas the (G/C) heterozygote were showed in both G and C allele. internal control at 446-bp product size.
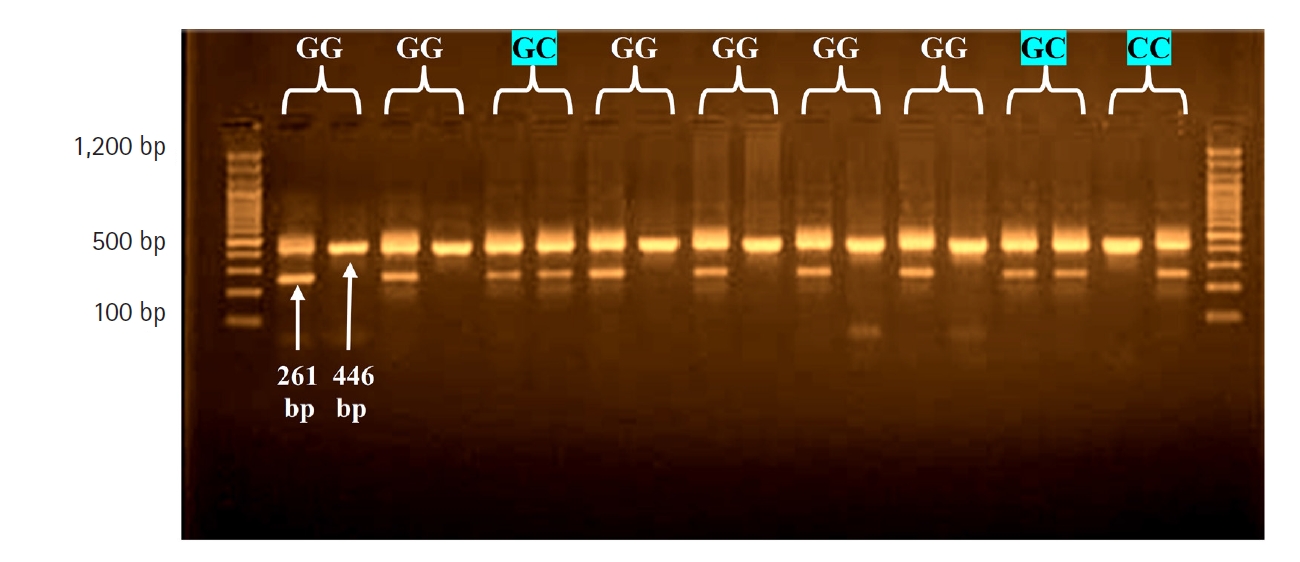
Table 1.
Primers for the IL18 â607C/A rs1946518 polymorphism
Table 2.
Primers for the IL18 â137G/C, rs187238 polymorphism
Table 3.
Hardy-Weinberg equilibriumâexpected genotype frequencies in IL18 rs1946518
| Groups | CC | CA | AA | C | A | Ď2 |
|---|---|---|---|---|---|---|
| Patient | ||||||
| genotypes | ||||||
| âObserved | 9 | 20 | 31 | 0.68 | 0.32 | 3.16a |
| âExpected | 6.0 | 26.0 | 28.0 | Not detected | - | |
| Control | ||||||
| genotypes | ||||||
| âObserved | 25 | 6 | 9 | 0.30 | 0.70 | 16.5a |
| âExpected | 19.6 | 16.8 | 3.6 | Not detected | - | |
Table 4.
Genotype distribution and allele frequency of IL18 rs1946518 (â607C/A) in breast cancer patients and controls with risk estimation
|
Study group |
OR (95% CI) | Fisherâs exact probability* | p-value | ||
|---|---|---|---|---|---|
| Patient (n = 60) | Control (n = 40) | ||||
| Genotype groups | |||||
| âC/C | 9 (15.0) | 25 (62.5) | 0.11 (0.04â0.28) | 0.000 | 0.0001*** |
| âC/A | 20 (33.3) | 6 (15.0) | 2.83 (1.02â7.86) | 0.061 | 0.040* |
| âA/A | 31 (51.7) | 9 (22.5) | 3.68 (1.49â9.04) | 0.003 | 0.004** |
| Allele distribution | |||||
| âC | 38 (31.7) | 56 (70.0) | 0.19 (0.11â0.36) | 0.000 | 0.0001*** |
| âA | 82 (68.3) | 24 (30.0) | 5.03 (2.72â9.30) | 0.000 | |
Table 5.
Hardy-Weinberg equilibriumâexpected genotype frequencies in IL18 rs187238
| Group | GG | GC | CC | G | C | Ď2 |
|---|---|---|---|---|---|---|
| Patientsâ | ||||||
| genotypes | ||||||
| âObserved | 20 | 26 | 14 | 0.45 | 0.55 | 0.93 NS |
| âExpected | 18.2 | 29.7 | 12.2 | Not detected | - | |
| Control | ||||||
| genotypes | ||||||
| âObserved | 26 | 9 | 5 | 0.24 | 0.76 | 5.74a |
| âExpected | 23.3 | 14.5 | 2.30 | Not detected | - | |
Table 6.
Genotype distribution and allele frequency of IL18 rs187238 (â137G/C) in breast cancer patients and controls with risk estimation
| Study group | OR (95% CI) | Fisherâs exact probability* | p-value | ||
|---|---|---|---|---|---|
| Patient (n = 60) | Control (n = 40) | ||||
| Genotype groups | |||||
| âG/G | 20 (33.3) | 26 (65.0) | 0.27 (0.12â0.62) | 0.002 | 0.002** |
| âG/C | 26 (43.3) | 9 (22.5) | 2.63 (1.07â6.48) | 0.035 | 0.04* |
| âC/C | 14 (23.3) | 5 (12.5) | 2.13 (0.70â6.47) | 0.203 | 0.180 NS |
| Alleles distribution | |||||
| âG | 66 (55.0) | 61 (76.2) | 0.38 (0.20â0.71) | 0.002 | 0.002** |
| âC | 54 (45.0) | 19 (23.8) | 2.63 (1.40â4.92) | 0.03 |
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209â249.



2. Breast Cancer Statistics and Resources | Breast Cancer Research Foundation | BCRF [Internet]. New York: Breast Cancer Research Foundation, 2022. Accessed 2022 Apr 3. Available from: https://www.bcrf.org/breast-cancer-statistics-and-resources/.
3. Liu Y, Lin N, Huang L, Xu Q, Pang G. Genetic polymorphisms of the interleukin-18 gene and risk of prostate cancer. DNA Cell Biol 2007;26:613â618.


5. Kaplanski G. Interleukin-18: biological properties and role in disease pathogenesis. Immunol Rev 2018;281:138â153.



6. Wawrocki S, Kielnierowski G, Rudnicka W, Seweryn M, Druszczynska M. Interleukin-18, functional IL-18 receptor and IL-18 binding protein expression in active and latent tuberculosis. Pathogens 2020;9:451.



7. Yang YC, Chang TY, Chen TC, Chang SC, Lin WS, Lee YJ. Genetic variants in interleukin-18 gene and risk for cervical squamous cell carcinoma. Hum Immunol 2013;74:882â887.


8. Giedraitis V, He B, Huang WX, Hillert J. Cloning and mutation analysis of the human IL-18 promoter: a possible role of polymorphisms in expression regulation. J Neuroimmunol 2001;112:146â152.


9. Arimitsu J, Hirano T, Higa S, Kawai M, Naka T, Ogata A, et al. IL-18 gene polymorphisms affect IL-18 production capability by monocytes. Biochem Biophys Res Commun 2006;342:1413â1416.


10. Jia Y, Zang A, Jiao S, Chen S, Yan F. The interleukin-18 gene promoter -607 A/C polymorphism contributes to non-small-cell lung cancer risk in a Chinese population. Onco Targets Ther 2016;9:1715â1719.



11. Khalili-Azad T, Razmkhah M, Ghiam AF, Doroudchi M, Talei AR, Mojtahedi Z, et al. Association of interleukin-18 gene promoter polymorphisms with breast cancer. Neoplasma 2009;56:22â25.


12. Taheri M, Hashemi M, Eskandari-Nasab E, Fazaeli A, Arababi F, Bahrani-Zeidabadi M, et al. Association of -607 C/A polymorphism of IL-18 gene (rs1946518) with breast cancer risk in Zahedan, Southeast Iran. Prague Med Rep 2012;113:217â222.


13. Back LK, Farias TD, da Cunha PA, Muniz YC, Ribeiro MC, Fernandes BL, et al. Functional polymorphisms of interleukin-18 gene and risk of breast cancer in a Brazilian population. Tissue Antigens 2014;84:229â233.


14. Almohaidi AM, Al-Abassi HM, Abdulkareem ZJ. Sera level and polymorphism of interleukin-33 gene in Iraqi females patients with breast cancer. Ibn AL-Haitham J Pure Appl Sci 2021;34:1â11.


15. Zhao Y, Wang S, Zhang Z, Qin C, Yang X, Xie Q. Association of IL-18 genetic polymorphisms and haplotypes with breast cancer risk in a Chinese population. Biomed Res 2017;28:8433â8437.
16. Li X, Ren D, Li Y, Xu J, Liu C, Zhao Y. Increased cancer risk associated with the -607C/A polymorphism in interleukin-18 gene promoter: an updated meta-analysis including 12,502 subjects. J BUON 2015;20:902â917.

17. Fathi Maroufi N, Aghayi E, Garshasbi H, Gholampour Matin M, Babazadeh Bedoustani A, Firouzi Amoudizaj F, et al. Association of rs1946518 C/A polymorphism in ppromoter region of interleukin 18 gene and breast cancer risk in Iranian women: a case-control study. Iran J Allergy Asthma Immunol 2019;18:671â678.

18. Dwivedi S, Goel A, Khattri S, Mandhani A, Sharma P, Misra S, et al. Genetic variability at promoters of IL-18 (pro-) and IL-10 (anti-) inflammatory gene affects susceptibility and their circulating serum levels: an explorative study of prostate cancer patients in North Indian populations. Cytokine 2015;74:117â122.


19. Andersen SW, Trentham-Dietz A, Gangnon RE, Hampton JM, Figueroa JD, Skinner HG, et al. Breast cancer susceptibility loci in association with age at menarche, age at natural menopause and the reproductive lifespan. Cancer Epidemiol Biomarkers Prev 2013;22:478.


20. Nikiteas N, Yannopoulos A, Chatzitheofylaktou A, Tsigris C. Heterozygosity for interleukin-18 -607 A/C polymorphism is associated with risk for colorectal cancer. Anticancer Res 2007;27:3849â3853.

21. Bushley AW, Ferrell R, McDuffie K, Terada KY, Carney ME, Thompson PJ, et al. Polymorphisms of interleukin (IL)-1alpha, IL-1beta, IL-6, IL-10, and IL-18 and the risk of ovarian cancer. Gynecol Oncol 2004;95:672â679.

22. Guo XG, Xia Y. The Interleukin-18 promoter -607C>A polymorphism contributes to nasopharyngeal carcinoma risk: evidence from a meta-analysis including 1,886 subjects. Asian Pac J Cancer Prev 2013;14:7577â7581.


23. Eissa SA, Zaki SA, El-Maghraby SM, Kadry DY. Importance of serum IL-18 and RANTES as markers for breast carcinoma progression. J Egypt Natl Canc Inst 2005;17:51â55.

24. Ye ZB, Ma T, Li H, Jin XL, Xu HM. Expression and significance of intratumoral interleukin-12 and interleukin-18 in human gastric carcinoma. World J Gastroenterol 2007;13:1747â1751.



25. Almohaidi AM, Salman NF, Mohammed AK, Abbas AS, Hasan DH. A study of the association of interleukin-18 (rs187238, -137g/c) polymorphism in a sample of Iraqi patients with type II diabetes mellitus. Biochem Cell Arch 2018;18:695â702.
- TOOLS
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 3,573 View
- 142 Download
- Related articles in GNI








