Analysis of Gene Expression in Cyclooxygenase-2-Overexpressed Human Osteosarcoma Cell Lines
Article information
Abstract
Osteosarcoma is the most common primary bone tumor, generally affecting young people. While the etiology of osteosarcoma has been largely unknown, recent studies have suggested that cyclooxygenase-2 (COX-2) plays a critical role in the proliferation, migration, and invasion of osteosarcoma cells. To understand the mechanism of action of COX-2 in the pathogenesis of osteosarcoma, we compared gene expression patterns between three stable COX-2-overexpressing cell lines and three control cell lines derived from U2OS human osteosarcoma cells. The data showed that 56 genes were upregulated, whereas 20 genes were downregulated, in COX-2-overexpressed cell lines, with an average fold-change > 1.5. Among the upregulated genes, COL1A1, COL5A2, FBN1, HOXD10, RUNX2, and TRAPPC2are involved in bone and skeletal system development, while DDR2, RAC2, RUNX2, and TSPAN31are involved in the positive regulation of cell proliferation. Among the downregulated genes, HIST1H1D, HIST1H2AI, HIST1H3H, and HIST1H4C are involved in nucleosome assembly and DNA packaging. These results may provide useful information to elucidate the molecular mechanism of the COX-2-mediated malignant phenotype in osteosarcoma.
Introduction
Osteosarcoma is the most common primary bone tumor, representing over 56% of all bone tumors. It generally affects young people with the age of 15 to 19 and usually occurs in long bones near the metaphyseal growth plates. It has a high tendency to metastasize, and about 10%-20% of patients have macroscopic evidence of metastasis at the time of diagnosis, while 80%-90% of patients are assumed to have micrometastasis. Although it has been postulated that rapid bone growth, exposure to radiation, and genetic predispositions, such as RB or p53 mutations, are thought to be risk factors for developing osteosarcoma, the etiology has not been fully understood [1, 2, 3].
Prostaglandin endoperoxide synthase 2 (PTGS2), also called as cyclooxygenase-2 (COX-2), catalyzes the convertsion of arachidonic acid to prostaglandin H2, from which various prostanoids, including prostaglandin E2, are produced [4].
Accumulating evidence indicates that COX-2 is involved in osteosarcoma development and progression. Several studies have reported that high levels of COX-2 expression is associated with advanced clinical stage and metastasis [5, 6], as well as with lower overall survival rates and disease-free survival rates [7, 8, 9]. In addition, COX-2 inhibition by using RNAi or antisense oligonucleotide inhibits cell proliferation and invasion in human osteosarcoma cells [10, 11]. Also, selective COX-2 inhibitors reduce not only osteosarcoma cell proliferation and invasion in vitro but also tumor growth and metastasis in vivo [12, 13]. Moreover, we have previously reported that COX-2 overexpression promotes cell proliferation, migration, and invasion in U2OS human osteosarcoma cells [14]. These studies strongly suggest that COX-2 might be a causal factor for the development and progression of osteosarcoma. However, the exact mechanisms of action of COX-2 in osteosarcoma are largely unknown.
In an attempt to figure out the mechanism of action of COX-2 in osteosarcoma, we analyzed the gene expression profiles in three COX-2-overexpressed U2OS stable cell lines and three control stable cell lines.
Methods
Establishment and maintenance of stable cell lines
Human COX-2 cDNA was subcloned into the pcDNA3 vector containing neor. U2OS cells were transfected with COX-2 or pcDNA3 DNA using Lipofectamine2000 (Life Technologies, Grand Island, NY, USA). Transfectants were selected in the presence of geneticin, and individual clones were maintained in Dulbecco's modified Eagle's medium, containing fetal bovine serum (10%), penicillin (100 units/mL), streptomycin (100 units/mL), and geneticin (700 µg/mL), as reported previously [14].
RNA isolation
Total RNA was extracted from cells with Trizol (Life Technologies), purified with the addition of chloroform, and precipitated with the addition of isopropanol. The RNA concentration was determined by a spectrophotometer, and the quality of RNA was evaluated by the OD 260/280 ratio and gel electrophoresis.
Hybridization to expression arrays
The following procedures were carried out by Macrogen Co. (Seoul, Korea). First, total RNA was amplified and purified using the Ambion Illumina RNA amplification kit to yield biotinylated cRNA (Ambion, Austin, TX, USA). Briefly, 550 ng of total RNA was reverse-transcribed to cDNA using a T7 oligo(dT) primer. Second-strand cDNA was synthesized, in vitro-transcribed, and labeled with biotin-NTP. After purification, 750 ng of labeled cRNA was hybridized to the humanHT-12 expression v.4 bead array (Illumina, San Diego, CA, USA) for 16-18 h at 58℃. The array signal was detected using Amersham fluorolink streptavidin-Cy3 (GE Healthcare Bio-Sciences, Little Chalfont, UK). Arrays were scanned with an Illumina bead array reader/confocal scanner. Array data were filtered by a detection p-value < 0.05 (similar to signal to noise). Selected gene signal values were log-transformed and normalized by the quantile method.
Statistical analysis
Basic statistical analyses were performed using Microsoft Excel. Hierarchical cluster analysis was conducted with normalized log2-gene expression values using Cluster 3.0, and the results were visualized using Java Treeview [15, 16]. An unrooted tree was drawn with R package. Biological function analysis was performed with official gene names using DAVID (http://david.abcc.ncifcrf.gov/).
Results
Stable cell lines
We have previously established stable cell lines over-expressing human COX-2 in U2OS human osteosarcoma cells. To avoid clonal variations, we established three stable COX-2-overexpressing cell lines (U2OS-COX-2 #1, #2, and #3) and three control cell lines (U2OS-pcDNA3 #1, #2, and #3). High levels of COX-2 expression were observed by western blot analysis in U2OS-COX-2 cells, whereas the expression was barely detectable in U2OS-pcDNA3 cells. When we cultured the stable cell lines, we observed that cell proliferation, migration, and invasion rates were increased significantly in U2OS-COX-2 cells as compared with U2OS-pcDNA3 cells [14] (Table 1).
Gene expression
We first performed unsupervised cluster analysis comparing COX-2-overexpressing cell lines and control cell lines. Among 24,371 probe sets, we selected and analyzed 567 probe sets with a standard deviation > 0.5. The group of U2OS-COX-2 (U2OS-COX-2 #1, #2, and #3) and the group of U2OS-pcDNA3 (U2OS-pcDNA3 #1, #2, and #3) were well segregated in the unsupervised cluster analysis, confirming that our experiment was validated (Fig. 1).
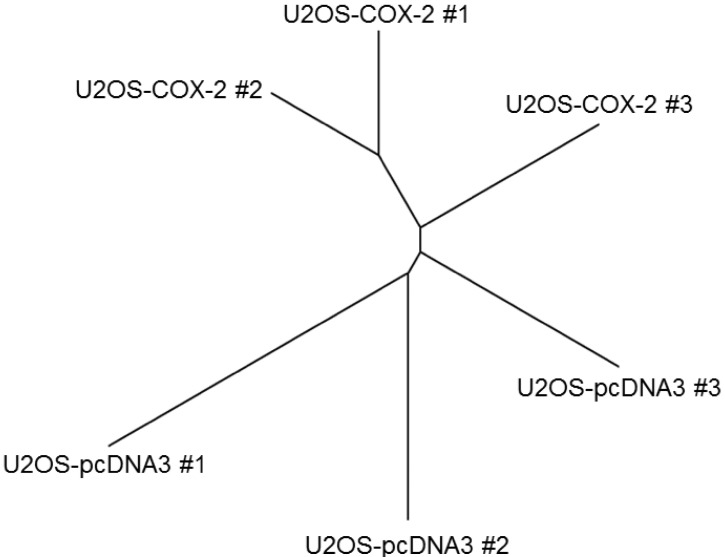
Segregation between the group of U2OS-COX-2 and the group of U2OS-pcDNA3. Unsupervised cluster analysis was done between six stable cell lines using 567 probe sets with a standard deviation > 0.5. Normalized log2-gene expression values were used for hierarchical clustering, and an unrooted tree was drawn by R package. COX-2, cyclooxygenase-2.
To identify significantly differentially expressed genes (DEGs) in COX-2-overexpressing osteosarcoma cells, we first performed the Wilcoxon rank sum test between the group of U2OS-COX-2 and the group of U2OS-pcDNA3. The 2,144 probe sets passed the criteria. Then, to identify DEGs, we selected 76 probe sets with an average fold-change > 1.5.
Among the DEGs, 56 genes were upregulated while 20 genes were downregulated in the group of U2OS-COX-2 as compared to the group of U2OS-pcDNA3 (Tables 2 and 3). The heat map of DEGs is provided in Fig. 2. The gene encoding COX-2, PTGS2, was upregulated by 10.41-fold in the COX-2-overexpressing cell lines as compared to the control cell lines, confirming again that our experiment is validated (Table 2).
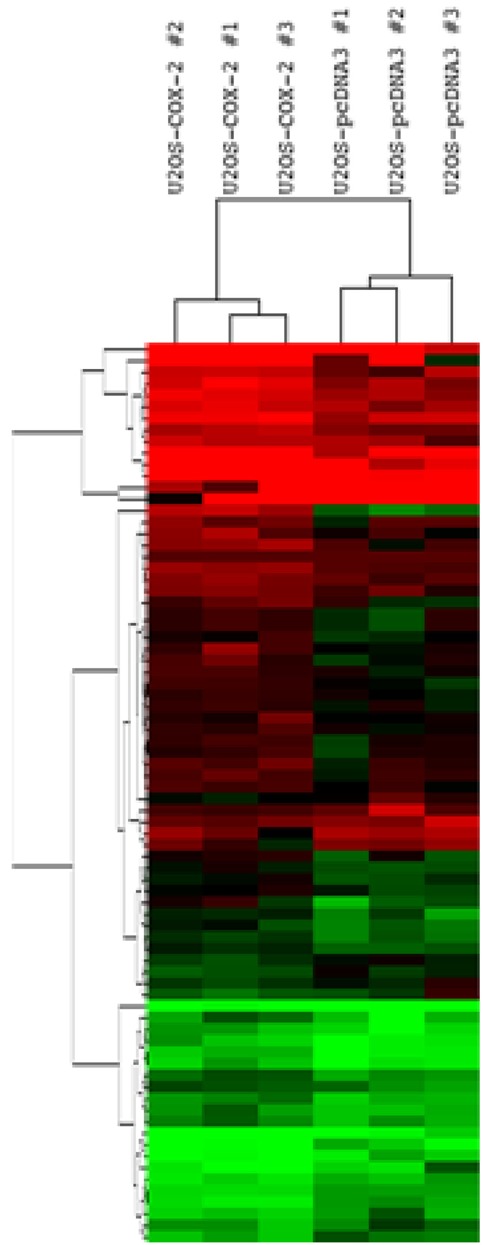
Gene expression patterns of 76 differentially expressed genes (DEGs). Hierarchical cluster analysis was done between six stable cell lines using 76 DEGs. Normalized log2-gene expression values were analyzed by Cluster 3.0, and the results were visualized using Java Treeview, with red being high and green indicating low.
Functional analysis
The DEGs were concentrated in several important biological functions
The functions that were most significantly upregulated by COX-2 overexpression in U2OS cells were skeletal system development and morphogenesis, bone development and morphogenesis, ossification, and positive regulation of cell proliferation (p < 0.05) (Table 4). The genes of COL1A1, COL5A2, FBN1, HOXD10, RUNX2, and TRAPPC2 are involved in skeletal system development and morphogenesis. The genes of COL1A1, COL5A2, and RUNX2 are involved in bone development, morphogenesis and ossification. The genes of DDR2, RAC2, RUNX2, and TSPAN31 are involved in the positive regulation of cell proliferation (Table 4).
The functions that were most significantly downregulated by COX-2 overexpression in U2OS cells were nucleosome assembly, chromatin assembly, and DNA packaging (p < 0.05). The genes of HIST1H1D, HIST1H2AI, HIST1H3H, and HIST1H4C are involved in these processes (Table 5).
Discussion
COX-2 has been reported to promote cell proliferation, migration, and invasion in osteosarcoma cells. COX-2 gene knockdown by RNAi significantly reduced the cell proliferation, migration, and invasion in SaOS2 cells [11], whereas stable expression of COX-2 increased the ability in U2OS cells [14] (Table 1). These studies demonstrated that abnormal COX-2 overexpression is an important determinant for the malignant phenotype of osteosarcoma cells. By using bioinformatics tools, we here provided useful information to understand the underlying mechanism by which COX-2 promotes the malignant phenotype in osteosarcoma cells.
According to our data, the genes encoding extracellular matrix proteins are highly upregulated by COX-2 (Tables 2 and 4). The genes COL1A1, COL5A2, and FBN1 encode α1 chain of type I collagen, α2 chain of type V collagen, and fibrillin 1, respectively. It has been known that the type I and type V collagens are major extracellular matrix proteins in bone [17]. Although there is a report that type I collagen promotes cell proliferation and migration in gastric cancer cells [18], the causal relationship between the overexpression of those genes and osteosarcoma has not been investigated well. Therefore, further studies are necessary to elucidate whether overexpression of these genes might be involved in the COX-2-mediated malignant phenotype in osteosarcoma.
Extracellular matrix proteins not only are structural components of the connective tissue in the body but also regulate cell fates through their receptors on the cell surface. DDR2 is one of the receptors for collagen, the expression of which was highly upregulated by COX-2 (Tables 2 and 4). DDR2 is a receptor tyrosine kinase, and its overexpression promotes the migration and invasion of cancer cells derived from breast, head and neck, and prostate [19, 20, 21]. Therefore, COX-2 might promote cell proliferation and motility through upregulation of DDR2. Further studies are necessary to elucidate this possibility.
RAC2, a highly upregulated gene by COX-2, is a member of the Rho family of GTPases (Tables 2 and 4). Although there is no report on Rac2 function in osteosarcoma, there is a report that Rac2 GTPase is required for B cell proliferation and survival [22]. Therefore, COX-2 might promote cell proliferation through upregulation of Rac2 function.
Among the highly upregulated genes, RUNX2 encodes a transcription factor governing osteoblast differentiation and skeletal morphogenesis. Recent studies on Runx2 function in cancer are controversial. Runx2 overexpression reduces cell proliferation in osteosarcoma cell lines [23] but promotes cell motility or invasion in MCF-7 breast cancer cells and PC prostate cancer cells [24, 25]. Therefore, further studies are required to elucidate the function of Runx2 in osteosarcoma and the relation between COX-2 and Runx2.
Intriguingly, the genes encoding histone proteins are highly downregulated by COX-2 (Tables 3 and 5). The genes HIST1H1D, HIST1H2AI, HIST1H3H, and HIST1H4C encode histone H1H1, histone H2A1, histone H3A1, and histone H41, respectively. As is well known, the nucleosome is the basic unit of DNA packaging, consisting of a core particle and linker DNA. A core particle comprises 147 base pairs of DNA wrapped around a histone octamer (2 copies each of histone H2A, H2B, H3, and H4). The core particles are connected by 10-90 bp of linker DNA, which could be naked or bound to histone H1 [26]. The nucleosome itself has been known to be a general gene repressor [27, 28]. In addition, RNAi-mediated knockdown of H2A.Z reduced cell proliferation in bladder cancer cells [29]. These results are compatible with our finding that COX-2 downregulates the expression of histone genes. Therefore, it is worth studying the mechanism of action of histones in osteosarcoma and the mechanism by which COX-2 downregulates histone genes.
Osteosarcoma is a highly malignant tumor, and the 5-year survival rate is only 20%-30% in patients with macroscopic evidence of metastasis at diagnosis [3]. While the etiology of osteosarcoma has been largely unknown, recent studies have suggested that COX-2 plays a critical role in osteosarcoma development and progression. In this regard, we believe that our study will provide useful information to understand the mechanism of action of COX-2 in osteosarcoma.
Acknowledgments
This study was supported by 2014 Research Grant from Kangwon National University (No. 120141490).