|
 |
- Search
| Genomics Inform > Volume 19(4); 2021 > Article |
|
Abstract
Campylobacter jejuni is one of the most prevalent organisms associated with foodborne illness across the globe causing campylobacteriosis and gastritis. Many proteins of C. jejuni are still unidentified. The purpose of this study was to determine the structure and function of a non-annotated hypothetical protein (HP) from C. jejuni. A number of properties like physiochemical characteristics, 3D structure, and functional annotation of the HP (accession No. CAG2129885.1) were predicted using various bioinformatics tools followed by further validation and quality assessment. Moreover, the protein-protein interactions and active site were obtained from the STRING and CASTp server, respectively. The hypothesized protein possesses various characteristics including an acidic pH, thermal stability, water solubility, and cytoplasmic distribution. While alpha-helix and random coil structures are the most prominent structural components of this protein, most of it is formed of helices and coils. Along with expected quality, the 3D model has been found to be novel. This study has identified the potential role of the HP in 2-methylcitric acid cycle and propionate catabolism. Furthermore, protein-protein interactions revealed several significant functional partners. The in-silico characterization of this protein will assist to understand its molecular mechanism of action better. The methodology of this study would also serve as the basis for additional research into proteomic and genomic data for functional potential identification.
As a human diarrheal pathogen Campylobacter jejuni, a well-known gram-negative bacterium, was first identified in 1973 [1]. It has several features like thermophilic, microaerophilic, no fermenting, non-spore forming, motile, single flagellum properties [2]. C. jejuni is a common foodborne pathogen that causes acute gastroenteritis in people globally and is prevalent in developed countries [1,3]. The incidence of infection by C. jejuni is more frequent than the infections caused by other common species including Escherichia coli O157:H7, Salmonella and Shigella [4]. C. jejuni possesses remarkable distinctive biochemical features from other microbial species including alpha-hemolysis, catalase sensitivity, hippurate hydrolysis, and nitrate reduction [5].
The genome of C. jejuni is made up of 1,641,481 base pairs containing 1,707 genes which are predicted to encode 1,654 proteins [6]. The functions of several of these proteins are still unknown. Uncharacterized protein families and domains of unknown functions both include proteins with uncertain functions [7]. For these reasons, the research interest for several unknown proteins of C. jejuni has increased among biological researchers. These unknown proteins, originated from an open reading frame with no experimental evidence of translation, are termed as hypothetical proteins (HPs) due to lack of functional annotations [8].
Over the last few decades a revolution in computational biology has led to the development of numerous servers and tools to aid in the prediction of protein function. HPs that have unknown features can be identified by virtue of their homology to known proteins [7]. A number of bioinformatics tools including the CD Search Service, InterProScan have been designed to specify functions of HPs from many bacterial species [9]. Furthermore, the study of protein-protein interaction (PPI), which play an essential role during cellular processes, is crucial to understand the function of a protein in a biological network using software such as the STRING database [10]. Three-dimensional (3D) modeling, however, is also important to correlate structural knowledge with the function of undetermined proteins, through homology searches at the Protein Data Bank [11].
The aim of this study was to ascribe structural and biological function of the HP NVI_CJUN_00861 (accession No. CAG2129885.1) of C. jejuni, involved in catabolism of a short chain fatty acid (SCFA). Among SCFAs found within the gut, C. jejuni metabolizes only acetate and lactate [12]. Therefore, a protein involved in metabolism of a SCFA will provide insight about the metabolic flexibility of C. jejuni. A number of in-silico techniques were used to predict the physicochemical properties, phylogenetic information, subcellular distribution, secondary and 3D structure, active site location, functional properties, and PPI of the HP engaged in metabolism.
The amino acid sequence of the HP (accession No. CAG2129885) from the bacteria Campylobacter jejuni was retrieved as FASTA format from the NCBI protein database (https://www.ncbi.nlm.nih.gov). We have reviewed all bioinformatics tools and databases used in this study for functional annotation of HP (Table 1). To analyze the sequence similarity, BlastP [13] was used. The MUSCLE v3.6 [14] was used to conduct multiple sequence alignment and MEGA X [15] to phylogenetic analysis.
The ProtParam (http://web.expasy.org/protparam) [16] tool of the ExPASy server was used to analyze the physicochemical properties of the protein. The ProtParam tool computes various physicochemical properties such as molecular weight, theoretical isoelectric point (pI), composition of amino acid, total number of positive and negative residues, instability index, aliphatic index (AI), grand average of hydropathicity (GRAVY), molecular formula, and estimated half-life.
The subcellular localization was anticipated by utilizing the CELLO server (http://cello.life.nctu.edu.tw) [17]. The results were further cross-checked by using PSLpred (http://crdd.osdd.net/raghava/pslpred) [18] and PSORTb (https://www.psort.org/psortb) [19] servers which are used to predict subcellular localization of bacterial proteins.
The Self-Optimized Prediction Method with Alignment server- SOPMA (https://npsa-prabi.ibcp.fr/cgibin/npsa_automat.pl?page=npsa_sopma.html) [20] was used to predict the studied protein's secondary structure. The result was cross-checked by using PSI-blast based secondary structure predicting PSIPRED server (https://bioinf.cs.ucl.ac.uk/psipred) [21].
The 3D model of the protein was generated by the HHpred server (https://toolkit.tuebingen.mpg.de/tools/hhpred) [22]. The YASARA energy minimization server (http://www.yasara.org/minimizationserver.htm) [23] was utilized to increase the side-chain accuracy, physical realism, and stereochemistry in homology modeling. The PyMOL v2.0 [24] was used for structural analysis and model figure generation. The SAVES server (https://services.mbi.ucla.edu) was used to assess the HP's anticipated 3D structure model's reliability. The Ramachandran plot analysis [25] in PROCHECK was used to visualize the backbone dihedral angles ψ against φ of amino acid residues in the HP structure, Verify3D [26] to determine the compatibility of an atomic model (3D) with its amino acid sequence, and ERRAT [27] to cross-check the studied HP structure.
To identify the conserved domain of the protein sequence, the Conserved Domain Search Service (CD Search) (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) [28] from NCBI was used. The protein sequence analysis and classification server InterProScan (https://www.ebi.ac.uk/interpro/search/sequence) [29] was then used for the functional analysis of the protein.
PPIs are a never-ending, intricate web of reactions that are essential for the control and execution of most biological processes. A protein-protein functional interaction network was identified by the STRING v11.0 (https://string-db.org) [10] search.
The active site of the HP was identified by the Computed Atlas of Surface Topography of Protein (CASTp) (http://sts.bioengr.uic.edu/castp) [30] which is an online asset for finding, outlining, and estimating inward surface regions on protein 3D structure.
A receiver operating characteristic (ROC) was carried out for randomly selected 40 proteins with known functions of C. jejuni (Supplementary Table 1) to confirm the accuracy of the predicted function of the HP using the same bioinformatics tools and databases that were used. We used two binary numerals “0” and “1” to classify the prediction as true positive (1) and true negative (0) whereas the integers (2, 3, 4, and 5) to evaluate the six levels diagnostic efficacy. The classification data were submitted to a web-based calculator to calculate the sensitivity, specificity, ROC area, and accuracy of the tools used to annotate the function of HP [31].
All information of the HP (accession No. CAG2129885) was collected from the NCBI database (Supplementary Table 2). BlastP was performed against the non-redundant protein sequences (nr) database and UniProtKB/Swiss-Prot (swissprot) database which showed demonstrated homology of the HP with other MmgE/PrpD family protein and cis-aconitate decarboxylase (CAD), respectively (Tables 2 and 3). A phylogenetic tree (Fig. 1) was constructed using the neighbor-joining method with a bootstrap replication of 1,000 to confirm the homology assessment between proteins.
The physicochemical properties of the studied protein (Supplementary Table 3) were obtained from the ExPASy ProtPram server illustrated that the protein contains 446 amino acids with a molecular weight of 49478.88 Da. Among the composition Ala (46), Ile (42), Leu (42), Lys (38), Phe (33), Ser (31), Asn (30), Glu (29), Gly (26), Asp (24), Val (17), Thr (14), Pro (14), His (13), Tyr (11), Gln (10), Cys (8), Met (8), Arg (7), and Trp (3) were most abundant. The number of negatively charged residues (Asp + Glu) and positively charged residues (Arg + Lys) was computed as 53 and 45, respectively. The pI was calculated as 5.93, which is an indicator that the protein is acidic (pH < 7) in character. The instability index was found to be 29.84 which classifies the HP as a stable protein. The AI was found to be 94.82 which implies the stability of the protein over a wide range of temperatures. The GRAVY score, ‒0.002, indicated that the protein is soluble in water (hydrophilic). The molecular formula of the HP was C2250H3490N574O649S16. The putative protein's half-life was estimated to be >20 h in yeast (in-vivo), >10 h in Escherichia coli (in-vivo), and 30 h in mammalian reticulocytes (in-vitro).
Since protein subcellular localization can provide information about a protein's function in an organism, computerized prediction of protein subcellular localization is an important technique for protein analysis and annotation. Subcellular localization involves the identification of the protein location within a cell. The protein functions are greatly influenced by their subcellular localization. Based on analysis of the CELLO server protein localization predictions, the HP was identified as a cytoplasmic protein. The PSORTb server also identified the protein as a cytoplasmic one with a high localization score (9.97). The PSLpred protein subcellular localization server similarly indicated the protein as a cytoplasmic one.
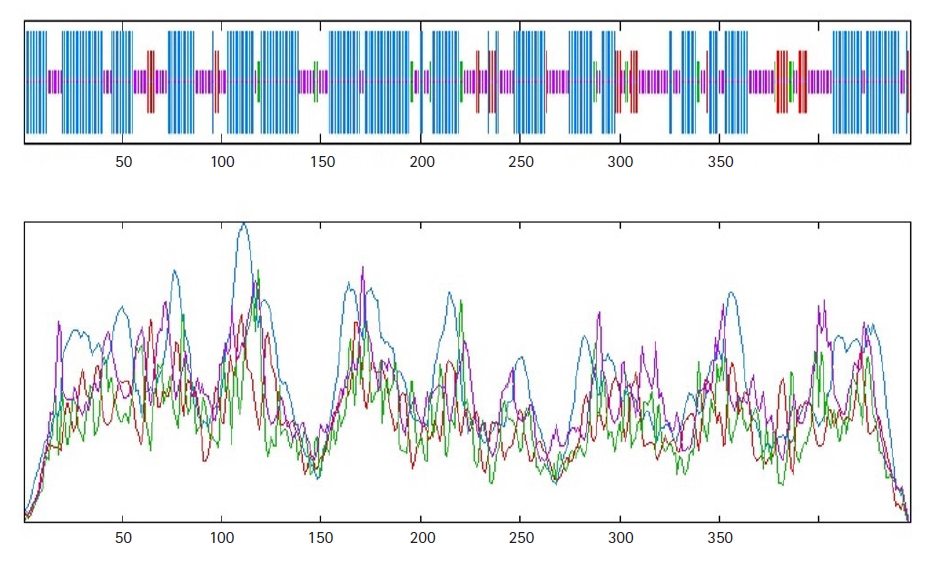
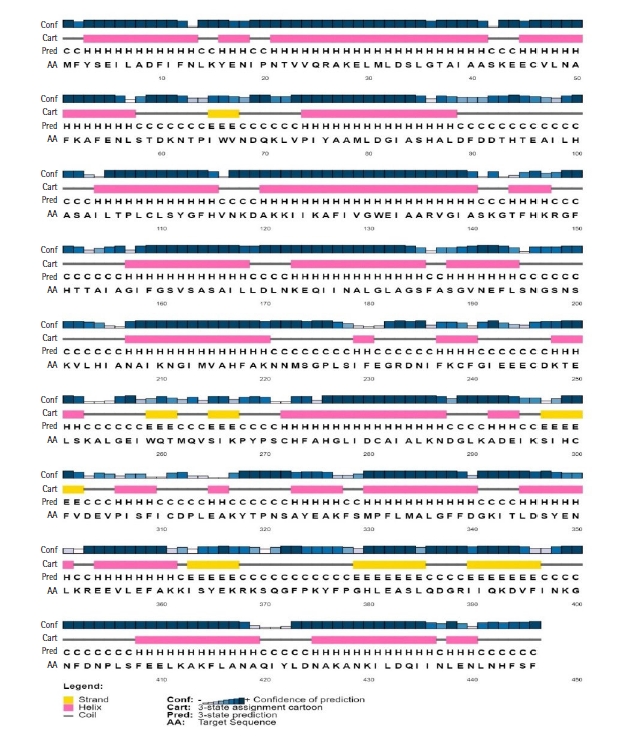
Protein function is highly conserved by its structure. A significant portion of the secondary structure of the protein is helix, sheet, turn, and coil. The secondary structure of the HP, obtained from SOPMA server, demonstrated that it was composed of the alpha helix (55.16%), random coil (33.41%), extended strand (7.17%), and beta-turn (4.26%) (Fig. 2). A similar result was found from the PSIPRED server (Fig. 3) validated the previous result.
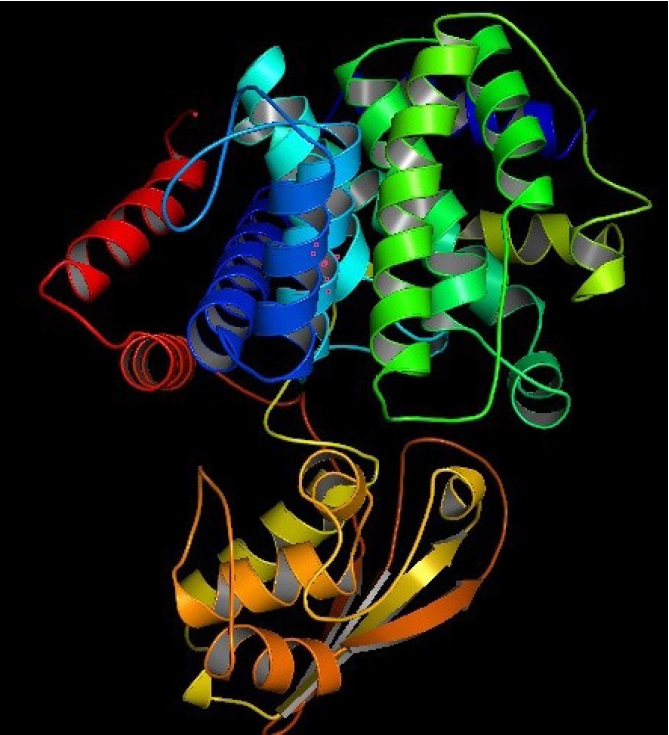
The 3D structure of a protein is intimately connected to its functional activities. Homology modeling was used to obtain the 3D structure of the HP from HHpred. YASARA energy minimization server modified the model structure to a more stable one by reducing its energy from 11,240.6 kJ/mol to ‒219,800.0 kJ/mol. The 3D structure of the protein obtained from PyMOL (Fig. 4) was validated by PROCHECK’s Ramachandran plot analysis, Verify3D, and ERRAT. The Ramachandran plot analysis (Fig. 5A) revealed that the number of amino acids in the most favored region was 91.3% (Supplementary Table 4), which is an indicator of a valid model. An overall quality factor of 96.99 by ERRAT verified the model as good quality (Fig. 5B). Verify3D also proved the validity of the predicted model by showing that 86.52% of the residues have averaged 3D-1D score ≥ 0.2 (Fig. 5C).
The conserved domain search service tool of NCBI had identified a functional domain located in the protein sequence of the HP. The domain that was found in the HP is of MmgE/PrpD family protein (accession No. pfam03972) which is involved in propionate catabolism. Under certain conditions, the breakdown of propionate results in the creation of propionyl-CoA, which is carboxylated to D-methylmalonyl-CoA, isomerized to L-methylmalonyl-CoA, and convertes to succinyl-CoA, which is supplied to various cellular processes [32]. Many bacteria can use propionate as their only carbon source. It has a close relationship with the malonate metabolic pathway and central metabolism [33].
The result was cross-checked by InterProScan and later validated by Pfam which produced the same result. Pfam server identified MmgE/PrpD N-terminal domain at 4-440 amino acid residues with an e-value of 3.8e-105. Additionally, to identify the accuracy of the tools and databases used to specify the function of the protein, ROC curve was calculated. An average accuracy for the used pipeline was found to be 96.7% and area under the curve was 0.99 (Table 4) indicating the high reliability of in-silico tools and databases used in this study.
Proteins belonging to the MmgE/PrpD family protein contain 2-methylcitrate dehydratase (PrpD; 4.2.1.79). The 2-methylcitric acid cycle catalyzed by PrpD leads to propionate catabolism. PrpD catalyzes the third step of the 2-methylcitric acid cycle [34,35]. This functional protein is made up of a broad domain with an all-helical fold and a smaller domain that folds into an alpha + beta domain [36]. CAD and MmgE/PrpD family protein share a lot of similarities. In Aspergillus terreus, CAD is needed for the production of itaconic acid [37]. It has been previously reported that citrate/2-methylcitrate dehydratase of Bacillus subtilis possesses both 2-methylcitrate dehydratase and citrate dehydratase and thus it is active in the tricarboxylic acid cycle and methylcitric acid processes [38].
PPI network of the HP was obtained from STRING server (Fig. 6). Functional partners with their scores predicted by the STRING search were gltA (0.991), acnB (0.961), purB-2 (0.886), metC (0.857), EAQ72564.1 (0.811), EAQ72574.1 (0.810), lecC (0.595), EAQ72769.1 (0.535), guaB (0.529), and acs (0.478) (Supplementary Table 5).
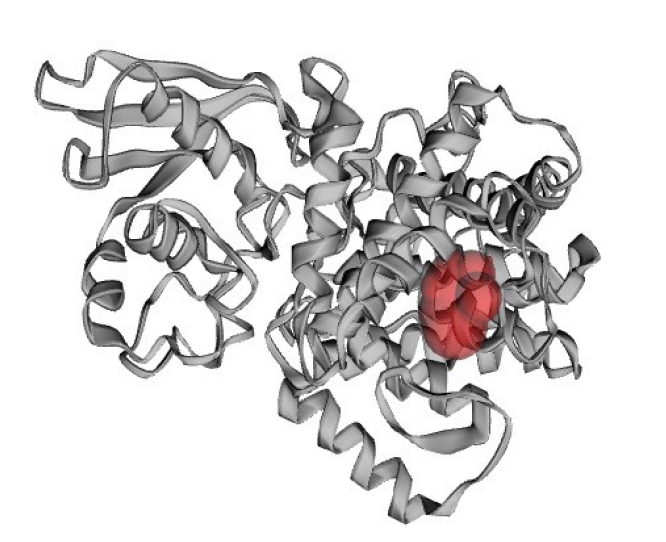
Protein’s active site is the region of its surface that facilitates its binding with a specific molecular substrate which then undergoes catalysis. The CASTp server had demonstrated that 14 amino acid residues were present in the active site of the protein (Fig. 7) and the best active site was in areas with 128.249 and a volume of 79.033. The residues in the active site were shown in Fig. 8.
Protein has a fundamental role in different biological processes, and all living things rely on it. The studied HP helps bacteria in propionate catabolism and influences the 2-methylcitric acid cycle. The basic knowledge on C. jejuni will be strengthened by these characters of the HP. However, the findings of the analyses show the validity of the bioinformatics tools and databases employed in this study, as well as the potential for extended in-vitro research on the HP.
Notes
Supplementary Materials
Supplementary data can be found with this article online at http://www.genominfo.org.
Supplementary Table 1.
List of annotated functions of 40 proteins with known function from Campylobacter jejuni using BLAST, CD Search, and InterProScan for ROC analysis
Supplementary Table 3.
Physicochemical properties of the hypothetical protein NVI_CJUN_00861
Supplementary Table 5.
Functional partners and their functions of the hypothetical protein predicted by the STRING server
Fig. 1.
Phylogenetic relatedness of the study protein (indicated with a black diamond) along with similar other proteins obtained from non-redundant protein sequences (nr) database. Scale bars represents substitutions per nucleotide site. Evolutionary analyses were conducted in MEGA X using Jones-Taylor-Thornton model with 1,000 bootstraps.

Fig. 5.
3D model of the studied hypothetical protein of Campylobacter jejuni validated by Ramachandran plot of PROCHECK program (A), ERRAT (B) (value overall quality factor: 96.991 from the SAVES server), and Verify3D (C).
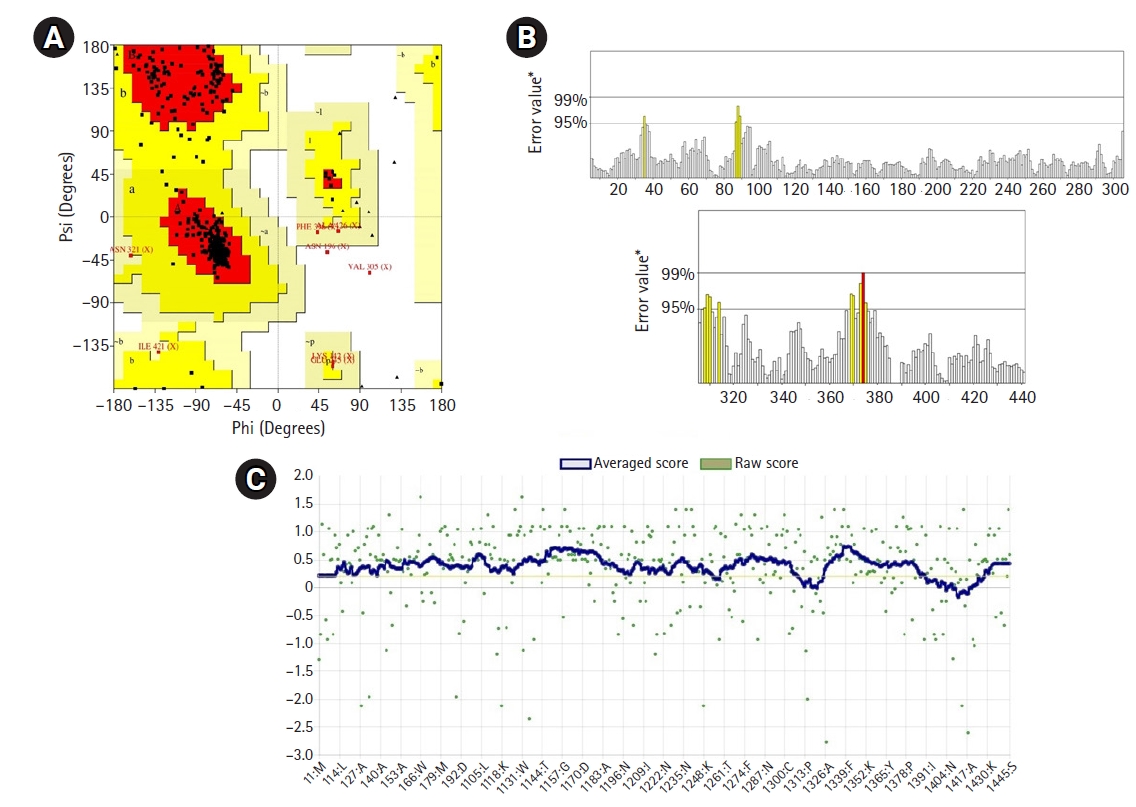
Fig. 6.
Protein-protein interaction network of the hypothetical protein from the STRING server. The colored nodes indicate the query proteins and the first shell of interactors, the white nodes indicate the second shell of interactors, the empty nodes represent proteins with an unknown three-dimensional structure, and the filled nodes represent proteins with a known or predicted three-dimensional structure.

Table 1.
List of bioinformatics tools and databases used for sequence based function annotation
| Sl | Software | Function | References |
|---|---|---|---|
| A | Sequence similarity search | ||
| 1 | BlastP | Used to find similar sequences in protein databases | [13] |
| 2 | MUSCLE | Used to conduct multiple sequence alignment | [14] |
| 3 | MEGA X | Used for inferring phylogenetic trees | [15] |
| B | Physiochemical characterization | ||
| 4 | ExPASy-Protparam tool | Used for computation of various physical and chemical parameters of protein | [16] |
| C | Sub-cellular localization | ||
| 5 | CELLO | Assign localization to both prokaryotic and eukaryotic proteins | [17] |
| 6 | PSLpred | Used to predict subcellular localization of proteins from Gram-negative bacteria | [18] |
| 7 | PSORTb | Used to predict subcellular localization of bacterial proteins | [19] |
| D | Secondary structure prediction | ||
| 8 | SOPMA | Used to predict the secondary structure of protein | [20] |
| 9 | PSIPRED | Used for predicting PSI-blast based secondary structure to analyze protein | [21] |
| E | 3D structure prediction and quality assessment | ||
| 10 | HHpred | Used to detect protein homology by HMM-HMM comparison | [22] |
| 11 | YASARA | Utilized to increase the stability of the 3D model structure | [23] |
| 12 | PyMOL | Used for structural analysis and model figure generation | [24] |
| 13 | PROCHECK’s Ramachandran plot analysis | Used to analyze the quality and accuracy of the predicted 3D model structure | [25] |
| 14 | Verify3D | Used to assess protein’s model with 3D profiles | [26] |
| 15 | ERRAT | Used to analyze the statistics of non-bonded interactions between different atoms and verify protein structures | [27] |
| F | Functional annotation | ||
| 16 | CD Search | Used to search for conserved structural and functional domains in a sequence | [28] |
| 17 | InterProScan | Used to search interPro for motif discovery | [29] |
| G | Protein-protein interaction | ||
| 18 | STRING | Used for predicting protein-protein interaction | [10] |
| H | Active site identification | ||
| 19 | CASTp | Used to find, outline, and estimate inward surface regions on protein 3D structure | [30] |
Table 2.
Similar protein obtained from non-redundant protein sequences (nr) database
Table 3.
Similar protein obtained from UniProtKB/Swiss-Prot (swissprot) database
References
1. Altekruse SF, Stern NJ, Fields PI, Swerdlow DL. Campylobacter jejuni: an emerging foodborne pathogen. Emerg Infect Dis 1999;5:28–35.



2. Balaban M, Hendrixson DR. Polar flagellar biosynthesis and a regulator of flagellar number influence spatial parameters of cell division in Campylobacter jejuni. PLoS Pathog 2011;7:e1002420.



3. Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol 2007;5:665–679.


4. Allos BM. Campylobacter jejuni Infections: update on emerging issues and trends. Clin Infect Dis 2001;32:1201–1206.


5. Snelling WJ, Matsuda M, Moore JE, Dooley JS. Campylobacter jejuni. Lett Appl Microbiol 2005;41:297–302.


6. Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, et al. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 2000;403:665–668.


7. Jaroszewski L, Li Z, Krishna SS, Bakolitsa C, Wooley J, Deacon AM, et al. Exploration of uncharted regions of the protein universe. PLoS Biol 2009;7:e1000205.



8. Nimrod G, Schushan M, Steinberg DM, Ben-Tal N. Detection of functionally important regions in "hypothetical proteins" of known structure. Structure 2008;16:1755–1763.


9. Ferdous N, Reza MN, Emon MT, Islam MS, Mohiuddin AK, Hossain MU. Molecular characterization and functional annotation of a hypothetical protein (SCO0618) of Streptomyces coelicolor A3(2). Genomics Inform 2020;18:e28.



10. Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 2019;47:D607–D613.


11. Jez JM. Revisiting protein structure, function, and evolution in the genomic era. J Invertebr Pathol 2017;142:11–15.


12. Stahl M, Butcher J, Stintzi A. Nutrient acquisition and metabolism by Campylobacter jejuni. Front Cell Infect Microbiol 2012;2:5.



13. Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 1997;25:3389–3402.



14. Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 2004;32:1792–1797.



15. Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 2018;35:1547–1549.



16. Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 2003;31:3784–3788.



17. Yu CS, Lin CJ, Hwang JK. Predicting subcellular localization of proteins for Gram-negative bacteria by support vector machines based on n-peptide compositions. Protein Sci 2004;13:1402–1406.



18. Bhasin M, Garg A, Raghava GP. PSLpred: prediction of subcellular localization of bacterial proteins. Bioinformatics 2005;21:2522–2524.


19. Yu NY, Wagner JR, Laird MR, Melli G, Rey S, Lo R, et al. PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 2010;26:1608–1615.



20. Secondary structure analysis of a protein using SOPMA. Ettimadai: Amrita Vishwa Vidyapeetham Virtual Lab, 2012. Accessed 2021 Nov 30. Available from: https://vlab.amrita.edu/?sub=3&brch=275&sim=1454&cnt=1.
21. Jones DT. Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol 1999;292:195–202.


22. Zimmermann L, Stephens A, Nam SZ, Rau D, Kubler J, Lozajic M, et al. A completely reimplemented MPI bioinformatics toolkit with a new HHpred server at its core. J Mol Biol 2018;430:2237–2243.


23. Krieger E, Joo K, Lee J, Lee J, Raman S, Thompson J, et al. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: four approaches that performed well in CASP8. Proteins 2009;77 Suppl 9:114–122.


24. Likova E, Petkov P, Ilieva N, Litov L. The PyMOL Molecular Graphics System, version 2.0. New York: Schrödinger, LLC, 2015.
25. Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 1993;26:283–291.

26. Eisenberg D, Luthy R, Bowie JU. VERIFY3D: assessment of protein models with three-dimensional profiles. Methods Enzymol 1997;277:396–404.

27. Colovos C, Yeates TO. Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci 1993;2:1511–1519.



28. Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, et al. CDD: a conserved domain database for the functional annotation of proteins. Nucleic Acids Res 2011;39:D225–D229.


29. Jones P, Binns D, Chang HY, Fraser M, Li W, McAnulla C, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics 2014;30:1236–1240.



30. Tian W, Chen C, Lei X, Zhao J, Liang J. CASTp 3.0: computed atlas of surface topography of proteins. Nucleic Acids Res 2018;46:W363–W367.



31. Eng J. ROC analysis: web-based calculator for ROC curves. Baltimore: Johns Hopkins University, 2014. Accessed 2021 Nov 30. Available from: http://www.jrocfit.org.
32. Halarnkar PP, Blomquist GJ. Comparative aspects of propionate metabolism. Comp Biochem Physiol B 1989;92:227–231.


33. Suvorova IA, Ravcheev DA, Gelfand MS. Regulation and evolution of malonate and propionate catabolism in proteobacteria. J Bacteriol 2012;194:3234–3240.



34. Horswill AR, Escalante-Semerena JC. In vitro conversion of propionate to pyruvate by Salmonella enterica enzymes: 2-methylcitrate dehydratase (PrpD) and aconitase enzymes catalyze the conversion of 2-methylcitrate to 2-methylisocitrate. Biochemistry 2001;40:4703–4713.


35. Blank L, Green J, Guest JR. AcnC of Escherichia coli is a 2-methylcitrate dehydratase (PrpD) that can use citrate and isocitrate as substrates. Microbiology (Reading) 2002;148:133–146.


36. Lohkamp B, Bauerle B, Rieger PG, Schneider G. Three-dimensional structure of iminodisuccinate epimerase defines the fold of the MmgE/PrpD protein family. J Mol Biol 2006;362:555–566.


- TOOLS
-
METRICS

-
- 6 Crossref
- 0 Scopus
- 6,246 View
- 196 Download
- Related articles in GNI