 |
 |
- Search
| Genomics Inform > Volume 21(4); 2023 > Article |
|
Abstract
Tumor hypoxia, oxygen deprivation state, occurs in most cancers and promotes angiogenesis, enhancing the potential for metastasis. The vascular endothelial growth factor (VEGF) family genes play crucial roles in tumorigenesis by promoting angiogenesis. To investigate the malignant processes triggered by hypoxia-induced angiogenesis across pan-cancers, we comprehensively analyzed the relationships between the expression of VEGF family genes and hypoxic microenvironment based on integrated bioinformatics methods. Our results suggest that the expression of VEGF family genes differs significantly among various cancers, highlighting their heterogeneity effect on human cancers. Across the 33 cancers, VEGFB and VEGFD showed the highest and lowest expression levels, respectively. The survival analysis showed that VEGFA and placental growth factor (PGF) were correlated with poor prognosis in many cancers, including kidney renal cell and liver hepatocellular carcinoma. VEGFC expression was positively correlated with glioma and stomach cancer. VEGFA and PGF showed distinct positive correlations with hypoxia scores in most cancers, indicating a potential correlation with tumor aggressiveness. The expression of miRNAs targeting VEGF family genes, including hsa-miR-130b-5p and hsa-miR-940, was positively correlated with hypoxia. In immune subtypes analysis, VEGFC was highly expressed in C3 (inflammatory) and C6 (transforming growth factor β dominant) across various cancers, indicating its potential role as a tumor promotor. VEGFC expression exhibited positive correlations with immune infiltration scores, suggesting low tumor purity. High expression of VEGFA and VEGFC showed favorable responses to various drugs, including BLU-667, which abrogates RET signaling, an oncogenic driver in liver and thyroid cancers. Our findings suggest potential roles of VEGF family genes in malignant processes related with hypoxia-induced angiogenesis.
Hypoxia is a state of oxygen deficiency commonly observed in most cancers. Tumor hypoxia causes the formation of blood vessels, enhancing the potential for metastasis. Previous studies revealed that hypoxia strongly induces vascular endothelial growth factor (VEGF) expression [1]. Angiogenesis is the process of creating new blood vessels from pre-existing vessels. Through this process, cells receive blood containing oxygen and nutrients for growth. Moreover, as the degree of angiogenesis increases in primary tumors, the prognosis worsens [2]. VEGF-VEGF receptors, well-known as key angiogenic factors, have been shown to play crucial roles in tumor initiation, progression, and metastasis. Despite ongoing efforts to investigate association between hypoxia and genes that promote angiogenesis, no studies have been conducted using VEGF family genes across pan-cancer.
The VEGF family comprises VEGFA, VEGFB, VEGFC, VEGFD, and placental growth factor (PGF), and they differ in function and expression. For a few cancers, overexpression of VEGF family genes and their correlation with the prognosis, metastasis, and recurrence have been reported. Compared to low expression, high VEGFA expression is associated with poor survival outcomes in gastric cancer [3], lung cancer [4], and colon cancer [5]. VEGFB facilitates tumor advancement by elevating plasminogen activators, which can lead to the metastasis of breast cancer [6]. The expression of VEGFC and VEGFD correlates with recurrence in head and neck squamous cell carcinomas [7] and lymphatic metastases in gastric cancer [8], respectively. Previous study reported that up-regulated PGF is associated with lymph node metastases and serosal invasion in gastric cancer [9].
Despite the potential involvement of the VEGF family genes in various tumor-related pathways, little is known of their roles in the tumor microenvironment (TME) and their impact on tumor hypoxia across pan-cancer. Therefore, the link between expression of the VEGF family genes and hypoxia, prognoses, immune subtypes, and pharmacological activity should be explored. Here, we comprehensively analyzed the expression patterns of VEGF family genes and their association with hypoxia scores, survival rates, immune subtypes, TME, and responses to chemotherapy in 33 cancers.
Pan-cancer data were downloaded (September 2022) from the UCSC Xena website (http://xena.ucsc.edu/). RNA-sequencing gene expression data (HTSeq-FPKM), micro-RNA (miRNA) expression data, survival data, clinical data, and immune subtypes were obtained from the Cancer Genome Atlas (TCGA) database. Data from TCGA comprised 11,057 samples from 33 cancers. The TCGA abbreviations and the detailed sample information, including tumor stage, and the number of tumor and normal samples, were summarized in Supplementary Table 1.
The average expression of VEGF family genes was estimated in 33 cancers with average of each cancer type. To compare the expression of VEGF family genes between tumors and normal tissues, we performed the Wilcoxon signed-rank test on 18 cancers which have more than five normal samples. The p-value was adjusted with Benjamini-Hochberg method and adjusted p-value < 0.05 was considered as significant. We estimated the expression correlation between the VEGF family genes using Spearman’s correlation method.
The patients were divided into two groups, high expression and low expression, based on the median expression level of each VEGF family gene. To analyze the prognostic differences between two groups, we used the “survival” and “survminer” R package to draw the Kaplan-Meier survival curves. The log-rank test was used to assess significance at p < 0.05. We explored the association between the expression of VEGF family genes and overall survival using univariate Cox proportional hazards regression. Moreover, the association between tumor stage and the expression of VEGF family genes was analyzed using the Kruskal-Wallis test and Benjamini-Hochberg p-value correction. Adjusted p-value < 0.05 was considered as significant.
Tumor hypoxia was quantified using published gene signatures in 20 cancers [10]. The mRNA data of the genes in the hypoxia signature were extracted from each cancer and combined as a single cohort to compare hypoxia across cancers. According to Bhandari et al. [10], cancers with mRNA abundance values in the top 50% for each gene signature were assigned a score of +1. Cancers with mRNA abundance values in the bottom 50% for that gene were assigned a score of –1. Using the Spearman correlation method, we demonstrated an association between hypoxia and the expression of VEGF family genes and miRNAs targeting them from all tumor samples of each cancer type.
Six immune subtypes, namely C1 (wound healing), C2 (interferon-γ dominant), C3 (inflammatory), C4 (lymphocyte-depleted), C5 (immunologically quiet), and C6 (transforming growth factor β [TGF-β] dominant), were identified using the global transcriptomic immune classification of solid tumors [11]. Because each immune subtype has various clinical and biological characteristics, understanding how the expression of VEGF family genes relates to the immune subtype affects cancer treatment determination is important. To analyze the relationship between the expression of VEGF family genes and the six immune subtypes, we performed differential expression analysis by using the Kruskal-Wallis test and Benjamini-Hochberg adjustment. Adjusted p < 0.05 indicated statistical significance.
To investigate the TME which consists of tumor cells and normal cells, such as stromal and immune, the ESTIMATE algorithm and Spearman’s correlation method were used to examine correlation between tumor purity and the expression of VEGF family genes from all tumor samples of each cancer type. By the ESTIMATE algorithm [12], the gene expression levels of a specific sample were normalized based on their ranks, and an enrichment score was generated by summing the difference of the empirical cumulative distribution functions (CDFs) of the signature genes and those of the remaining non-signature genes according to [13]. For a given signature S with nS genes and a single sample T, the n genes in the data set are assigned ranks based on their absolute expression levels and ordered by their rank from highest (nst) to lowest (1st), represented as L = {r1, r2, ..., rn}. An enrichment score ES(S, T) is obtained by summing the difference between the weighted empirical CDF of the signature genes P S w P S w
where P S w ( S , T , i ) = ∑ r j ∈ S , j ≤ 1 r j α ∑ r j ∈ S r j α P n S ( S , T , i ) = Σ r j ∉ S , j ≤ i 1 ( n - n S )
Transcripts and compound activity data were downloaded from the CellMiner database (https://discover.nci.nih.gov/cellminer/) that contains 60 human cancer cell lines (NCI-60). Raw data were processed using “impute” R package. Pearson’s correlation analysis was used to explore the correlation between the expression of VEGF family genes and drug sensitivity. Drugs used in clinical trials and Food and Drug Administration–approved drugs were included.
To understand the role of VEGF family genes in human cancers, we examined their expression patterns across 33 cancers. VEGFB has the highest gene expression level, and VEGFD has the lowest gene expression level (Fig. 1A). All expression pairs of VEGF family genes showed positive correlations, suggesting potential common features in biological functions and structures (Fig. 1B). The strongest positive correlation was observed between VEGFC and PGF (r = 0.34). Differential expression analysis of each VEGF family gene was performed in 18 cancers which have more than five normal samples. A higher expression of VEGF family genes, except VEGFD, was observed in tumor tissues than in normal tissues across most cancers (Fig. 1C–H). Notably, up-regulated VEGFA and PGF were observed in 15 cancers, with the highest differences observed in kidney renal clear cell carcinoma (KIRC) (Fig. 1C). The expression of VEGFD tended to be down-regulated in most cancers, except Cholangiocarcinoma (CHOL) and liver hepatocellular carcinoma (LIHC). Moreover, VEGFA, VEGFB, VEGFC, and PGF expression was significantly increased in CHOL, head and neck squamous cell carcinoma (HNSC), KIRC, and LIHC tumor tissues. LIHC was the only cancer with significant up-regulation of all VEGF family genes.
To predict whether the expression of VEGF family genes promotes or inhibits cancer, we performed a survival analysis in 33 cancers. Kaplan-Meier survival curves and univariate Cox proportional hazard ratio were used to investigate the relationship between the expression levels of VEGF family genes and patients' overall survival (Supplementary Table 2, Supplementary Fig. 1). Each VEGF family gene showed significant associations with cancer prognosis in at least four cancers (p < 0.05).
The expression of VEGFA, VEGFC, and PGF showed significant associations with poor prognosis in six different cancers (p < 0.05) (Supplementary Fig. 1): VEGFA played a damaging role in cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), colon adenocarcinoma (COAD), kidney renal papillary cell carcinoma (KIRP), brain lower grade glioma (LGG), LIHC, and prostate adenocarcinoma (PRAD); VEGFC played a damaging role in HNSC, LGG, lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), mesothelioma (MESO), and stomach adenocarcinoma (STAD); and PGF played a damaging role in adrenocortical carcinoma (ACC), KIRP, LIHC, Rectum adenocarcinoma (READ), STAD, and uveal melanoma (UVM). By contrast, the expression of VEGFB and VEGFD was related to a better prognosis across the three cancers: VEGFB was a favorable prognostic factor for esophageal carcinoma (ESCA), pancreatic adenocarcinoma (PAAD), and sarcoma (SARC); and VEGFD was a favorable prognostic factor for LUAD, PAAD, and SARC.
Significant prognostic differences were observed in LGG based on the expression levels of three VEGF family genes. In LGG, high expression of VEGFA and VEGFC was significantly associated with worse prognosis, while high PGF expression was significantly associated with a better prognosis.
Moreover, we analyzed the relationship between the tumor stage and the expression of VEGF family genes. Cancers exhibiting a statistically significant difference (adjusted p < 0.05) in at least three VEGF family genes are shown in Fig. 2. In KIRC, the expression of PGF was significantly associated with the tumor stage (Fig. 2A). In KIRP, VEGFA, VEGFC, and PGF were differentially expressed across tumor stages. Except for VEGFB and VEGFD, the genes showed an increasing tendency in gene expression from stages I to IV in KIRP (Fig. 2B). In STAD, the expression of VEGFB, VEGFC, and PGF showed the highest expression in stage II (Fig. 2C). In Thyroid carcinoma (THCA), most of the VEGF family genes, except VEGFC, were differentially expressed in different tumor stages (Fig. 2D). Therefore, the expression of VEGF family genes across different tumor stages may be used to estimate potential cancer progression.
Hypoxia, a precursor for angiogenesis, plays an important role in cancer progression and affects TME [14]. We investigated the correlations between the expression of VEGF family genes and hypoxia scores in 20 cancers (Fig. 3). High expression of VEGFA is positively correlated with hypoxia scores in most tumors, indicating a potential correlation with tumor aggressiveness. By contrast, the expression level of VEGFD is negatively correlated with the hypoxia score, suggesting VEGFD is mainly expressed during normoxia. Overall, VEGFA showed strong positive correlations with hypoxia compared to other VEGF family genes (Fig. 3A–E). VEGFC showed positive correlations with all hypoxia scores in LUAD (Fig. 3C). VEGFD showed strong negative correlations with most of the hypoxia scores in Bladder urothelial carcinoma (BLCA), LUAD, and PAAD (Fig. 3D). PGF showed positive correlations with hypoxia scores across most cancers (Fig. 3E).
Correlation analysis was performed to explore the association between the Buffa hypoxia score and the expression of miRNAs targeting VEGF family genes (Supplementary Fig. 2): Hsa-miR-101-3p targeting VEGFA and VEGFC was the only miRNA that showed a negative correlation with hypoxia in all cancers (Supplementary Fig. 2A and 2C); Hsa-miR-130b-5p targeting VEGFB showed positive correlations across most cancers, except for pheochromocytoma and paraganglioma (PCPG) and THCA (Supplementary Fig. 2B); Hsa-miR-940 targeting VEGFB was positively correlated with hypoxia in most cancers, except for BLCA and THCA; Hsa-miR-218-5p targeting VEGFC showed negative correlations with hypoxia in 18 cancers (Supplementary Fig. 2C); and hsa-miR-335-5p targeting VEGFD was positively correlated with hypoxia in 15 cancers (Supplementary Fig. 2D).
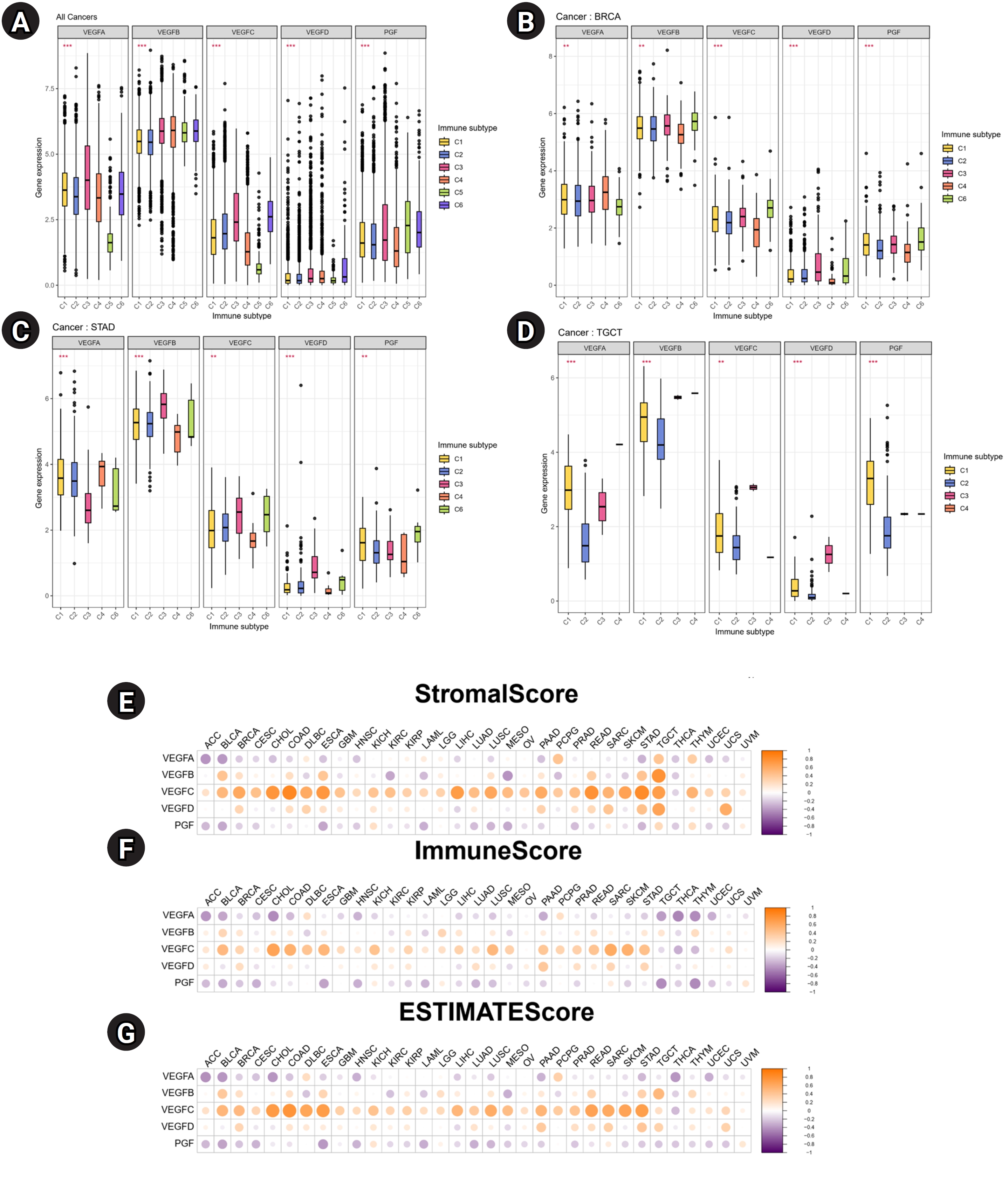
To identify the associations between the expression of VEGF family genes and immune components in tumor, correlation analysis was performed. Across multiple cancers, VEGFB was highly expressed in six immune subtypes than other VEGF family genes (Fig. 4A). In BRCA, VEGF family genes except VEGFA showed high expression in C3 and C6 (Fig. 4B). In STAD, VEGFD showed the highest expression in C3 (Fig. 4C). In testicular germ cell tumors (TGCT), all VEGF family genes showed the lowest expression in C2 (Fig. 4D). The TME plays crucial roles in tumor initiation, progression, and metastasis [15], so we investigated the relationship between the expression of VEGF family genes and the scores related to immune infiltration. Notable positive associations between stromal, immune, and ESTIMATE scores and VEGFC were observed in most cancers (Fig. 4E–G). VEGFA and PGF levels were inversely associated with these scores in most cancers.
To investigate the relationship between the responsiveness of more than 200 chemotherapy drugs in NCI60 cancer cell lines and the expression of VEGF family genes, correlation analysis was conducted (p < 0.01) (Supplementary Fig. 3). VEGFA expression was strongly and positively correlated with sensitivity to SAR-125844 (r = 0.66), abiraterone (r = 0.56), BLU-667 (r = 0.56), AZD-3229 (r = 0.56), and itraconazole (r = 0.46) (Supplementary Fig. 3A). VEGFC expression was distinctively correlated with good responses to JNJ-3387618 (r = 0.65), JNJ-38877605 (r = 0.57), staurosporine (r = 0.55), BLU-667 (r = 0.51), momelotinib (r = 0.51), dimethylfasudil (r = 0.5), zoledronate (r = 0.47), telatinib (r = 0.48), and dastinib (r = 0.47) (Supplementary Fig. 3B). 7–Hydroxystaurosporine showed a negative correlation with VEGFC expression (r = –0.35).
In this study, we aimed to explore the potential oncogenic roles of VEGF family genes across pan-cancer through sophisticated bioinformatic analyses. We observed heterogeneity in the expression patterns of VEGF family genes across different cancers. Except for CHOL and LIHC, VEGFD was down-regulated in 16 cancers. However, VEGFA and PGF were up-regulated in tumor tissues across most cancers including BRCA and HNSC (p < 0.001). The literature has reported that VEGFA expression was significantly increased in breast cancer and head and neck cancer [16,17].
In survival analysis, the expression of VEGF family genes was closely related to patients' survival; thus, these genes may be potential clinical prognostic indicators. Our findings showed the associations between the high expression of VEGFA, VEGFC, and PGF and poor prognosis across various cancers, suggesting that they may serve as risk factors for tumor progression. Increased expression of VEGFA and PGF was linked to poor prognosis in KIRP and LIHC. There is evidence that serum VEGFA levels are associated with poor prognosis in LIHC [18]. In addition, increased VEGFA and VEGFC were linked to poor prognosis in LGG. Another study revealed that inhibition of VEGFA prolongs the survival of patients with glioblastoma [19]. Furthermore, high expression of VEGFC and PGF was correlated with poor prognosis in STAD, supported by Li and Han [20]. We demonstrated an association between expression of VEGFB and VEGFD and favorable prognoses in multiple cancers, including PAAD and SARC. Notably, we are the first to demonstrate these results. Across tumor stages, VEGFA, VEGFC, and PGF exhibited an increasing expression tendency as the tumor stage advanced. In STAD, these genes showed a lower expression in stage I than in the other stages. Because the stage number indicates the extent of cancer spread, these results suggest that the expression of these genes is a clinicopathological marker.
Our investigation of this correlation with hypoxia provides insights into the potential role of VEGF family genes in driving malignant processes. For 20 cancers, the expression of VEGFA was positively associated, except for KIRC, PRAD, and THCA. This finding is supported by the literature indicating that hypoxia induces VEGFA expression [21]. Moreover, PGF exhibits a strong positive correlation with hypoxia in many cancers, including ovarian serous cystadenocarcinoma (OV). Previous study reported that epithelial ovarian cancer patients with high PGF expression show poor chemotherapy response and unfavorable prognosis [22]. We observed a distinct negative correlation between VEGFD expression and hypoxia across most cancers, but no study has established this finding.
Furthermore, we investigated the correlation between hypoxia scores and miRNAs targeting VEGF family genes. Hsa-miR-101-3p targeting VEGFA showed a negative correlation with hypoxia scores across most cancers, including LUAD and LUSC, suggesting its role as a tumor suppressor. This finding was supported by a finding in the literature that hsa-miR-101-3p inhibited VEGFA expression, which mediates invasion of lung cancer cells [23]. Hsa-miR-101-3p, which also targets VEGFC, was negatively correlated with hypoxia in LIHC. Another study revealed that hsa-miR-101-3p inhibited cell migration by suppressing VEGFC expression in hepatocellular carcinoma [24]. Moreover, hsa-miR-130b-5p targeting VEGFB exhibited a positive correlation with hypoxia in various cancers including BRCA and LUAD. Its involvement in hypoxia by regulating VEGFB has not been demonstrated. Consistent with the positive association between hsa-miR-940 expression and the hypoxia score shown in this study, hsa-miR-940 overexpression was correlated with tumor progression in gastric cancer, pancreatic carcinoma, and ovarian cancer [25-27]. We observed a negative correlation between hsa-miR-218-5p expression and hypoxia score in LUAD. Another study demonstrated that enhanced expression of hsa-miR-218-5p inhibited cell viability and migration in LUAD [28].
We observed that VEGF family genes were significantly correlated with the immune subtypes. In STAD, the expression of VEGFC and PGF was high in aggressive immune subtype C6 (TGF-β dominant), which is related to poor prognosis. These findings are consistent with our survival results, and the results of another study demonstrating that hypoxic tumor cells promote TGF-β activation, which has a tumor-promoting effect [29]. A consistently high expression of VEGFC in stromal, immune, and ESTIMATE scores indicated lower tumor purity. We observed that VEGFC was associated with poor prognosis across multiple cancers. Studies have demonstrated an association between low tumor purity and poor prognosis across various cancers [30,31]. The association between the VEGF family genes and drug response is also notable. Increased expression of VEGFA and VEGFC enhances sensitivity to various chemotherapeutic drugs.
Using integrated bioinformatics analyses, we comprehensively overviewed the roles of VEGF family genes in tumor progression and hypoxia. Because our findings were not validated by independent datasets, further validation using in vitro and in vivo experiments is necessary. The dynamics of gene families vary widely, so interpreting the overall pattern of positive correlations requires caution. Also, some VEGF family genes are included in multiple hypoxia signatures, which could lead to a bias in the correlation between hypoxia scores and expression level of VEGF family genes. Considering that the hypoxia scores were influenced by the expression levels of not only VEGFA but also other hypoxia-related genes, investigating the comprehensive relationship between the hypoxia scores and the VEGF family genes is not devoid of significance.
Overall, VEGFD exhibits opposite trend compared to other VEGF family genes. Thus, further research is needed to elucidate the directionality of VEGFD's behavior and its potential implications in hypoxia and other carcinogenic mechanisms.
In this study, we systematically investigated the expression of VEGF family genes and their relationships with patients' survival, hypoxic status, immune subtypes, and drug response in a pan-cancer analysis. Although our findings require further validation from laboratory results, we have provided a detailed overview of the biological functions of VEGF family genes in hypoxia. Thus, we provide insights into the role of VEGF family genes in cancers and provide blueprints for further research on their role in hypoxic TME.
Notes
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1C1C1012288).
Supplementary Materials
Supplementary data can be found with this article online at http://www.genominfo.org.
Supplementary Table 1.
The Cancer Genome Atlas (TCGA) pan-cancer data and abbreviations for the studied cancer types
Supplementary Table 2.
Univariate cox proportional hazard ratios of VEGF family genes according to gene expression across 33 cancers
Supplementary Fig. 1.
Kaplan-Meier survival curves comparing high and low expressions of vascular endothelial growth factor (VEGF) family genes (p < 0.05). Overall survival by VEGFA expression in different cancers: BLCA, CESC, COAD, KIRP, LGG, LIHC, and PRAD. Overall survival by VEGFB expression in different cancers: COAD, ESCA, PAAD, and SARC. Overall survival by VEGFC expression in different cancers: HNSC, LGG, LUAD, LUSC, MESO, PCPG, STAD, and UCEC. Overall survival by VEGFD expression in different cancers: LUAD, LIHC, PAAD, SARC, and THYM. Overall survival by PGF expression in different cancers: ACC, KIRP, LGG, LIHC, PRAD, READ, STAD, and UVM.
Supplementary Fig. 2.
Correlation between Buffa hypoxia score and the expression of miRNAs targeting each vascular endothelial growth factor (VEGF) family gene. miRNAs targeting (A) VEGFA, (B) VEGFB, (C) VEGFC, and (D) PGF.
Supplementary Fig. 3.
Correlation between drug response and vascular endothelial growth factor (VEGF) family genes in pan-cancer. (A) Correlations between VEGFA expression and sensitivity of SAR-125844, Abiraterone, BLU-667, AZD-3229, and itraconazole. (B) Correlations between VEGFC expression and sensitivity of JNJ-3887618, JNJ-38877605, staurosporine, BLU-667, momelotinib, dimethylfasudil, zoledronate, telatinib, dastinib, and 7-hydroxystaurosporine.
Fig. 1.
Expression patterns of vascular endothelial growth factor (VEGF) family genes. (A) Distribution of expression level of VEGF family genes across 33 cancers. (B) Associations between the expression of individual VEGF family genes. Pink and green indicate positive and negative correlations, respectively. (C) The differences in mean expression level of VEGF family genes between tumor and normal tissues. VEGFA (D), VEGFB, (E), VEGFC (F), VEGFD (G), and placental growth factor (PGF) (H) gene’s differential expression levels between tumor and normal tissues. BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CHOL, cholangiocarcinoma; COAD, colon adenocarcinoma; ESCA, esophageal carcinoma; GBM, glioblastoma multiforme; HNSC, head and neck squamous cell carcinoma; KICH, kidney chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; STAD, stomach adenocarcinoma; THCA, thyroid carcinoma; UCEC, uterine corpus endometrial carcinoma.. *Adjusted p < 0.05, **Adjusted p < 0.01, ***Adjusted p < 0.001.
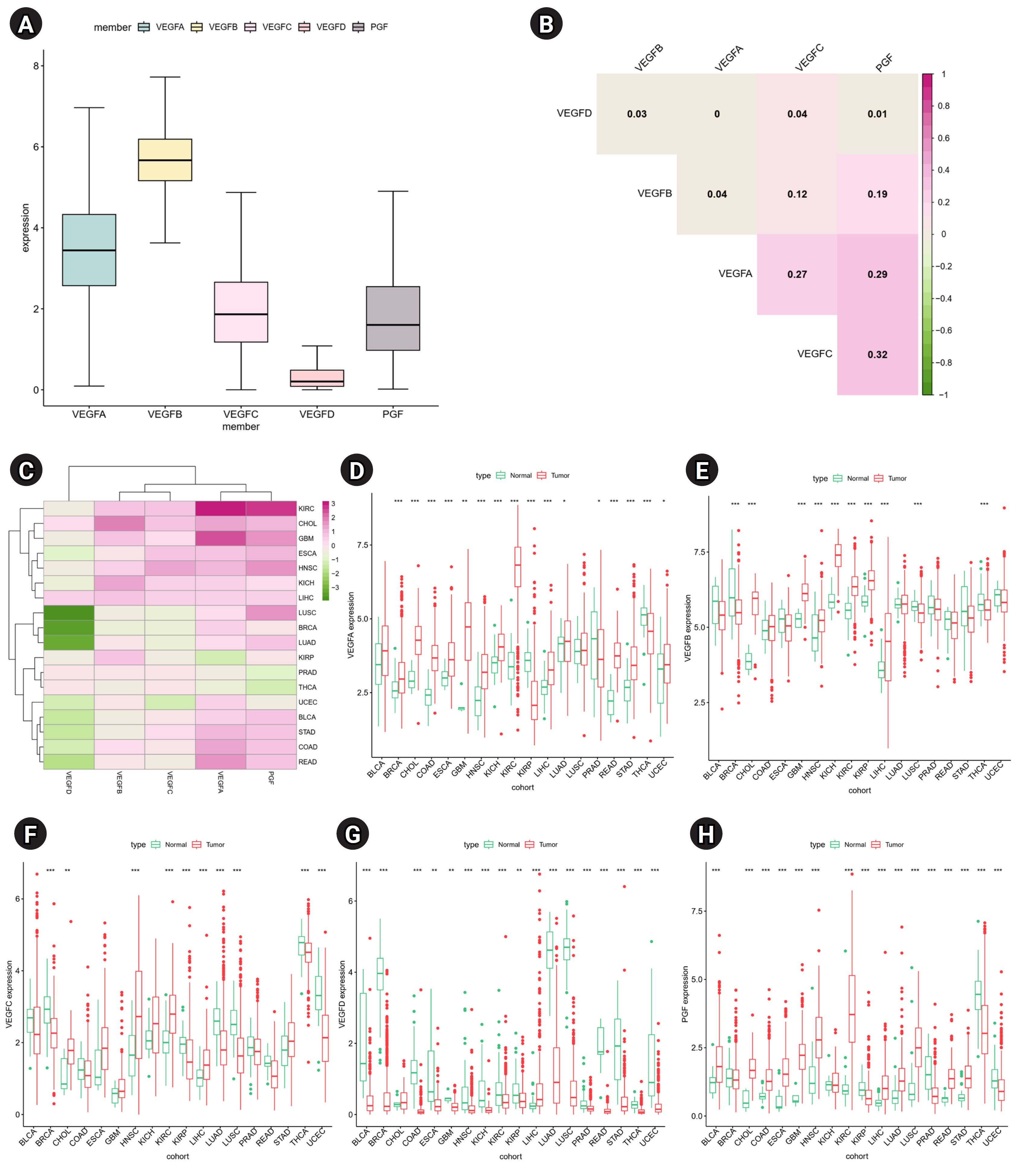
Fig. 2.
Correlations of vascular endothelial growth factor (VEGF) family genes with patients’ overall survival and tumor stages. Only cancers with p < 0.05 in at least three VEGF family genes are displayed: (A) kidney renal clear cell carcinoma (KIRC), (B) kidney renal papillary cell carcinoma (KIRP), (C) stomach adenocarcinoma (STAD), and (D) thyroid carcinoma (THCA). *Adjusted p < 0.05, **Adjusted p < 0.01, ***Adjusted p < 0.001.

Fig. 3.
Correlations between hypoxia scores and mRNA expression of vascular endothelial growth factor (VEGF) family genes: (A) VEGFA, (B) VEGFB, (C) VEGFC, (D) VEGFD, and (E) placental growth factor (PGF). BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; GBM, glioblastoma multiforme; HNSC, head and neck squamous cell carcinoma; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LGG, brain lower grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma; SKCM, skin cutaneous melanoma; THCA, thyroid carcinoma; UCEC, uterine corpus endometrial carcinoma; COAD, colon adenocarcinoma; READ, rectum adenocarcinoma.
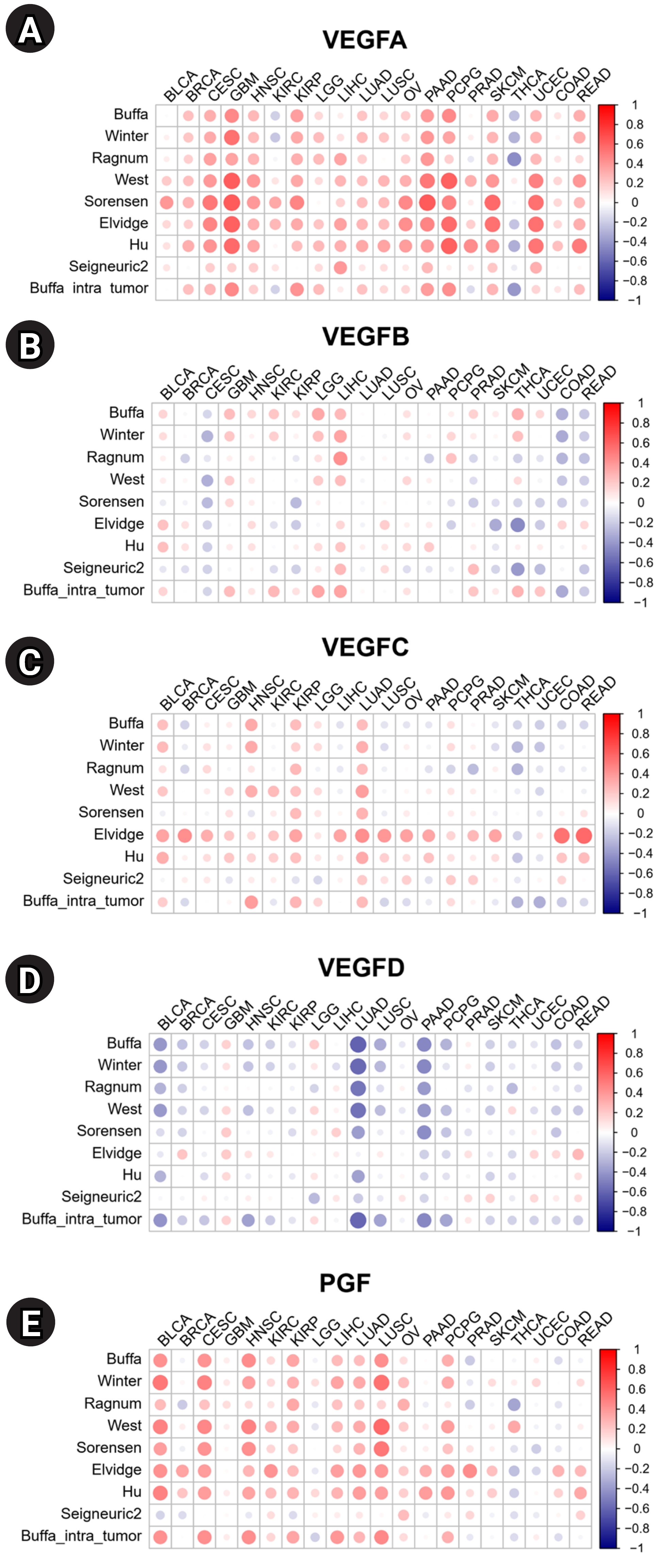
Fig. 4.
Correlations of vascular endothelial growth factor (VEGF) family genes with immune subtypes, cancer stemness score, and the tumor microenvironment in pan-cancer. (A) A Box plot showing the expression of VEGF family genes differed across pan-cancer. (B-D) The overall transcriptional expression level of VEGF family genes in (B) breast invasive carcinoma (BRCA), (C) stomach adenocarcinoma (STAD) and (D) testicular germ cell tumors (TGCT). *Adjusted p < 0.05; **Adjusted p < 0.01; ***Adjusted p < 0.001. (E-G) Correlation plots demonstrate associations between the mRNA expression of VEGF family genes and (E) Stromal score, (F) Immune score, and (G) ESTIMATE score calculated by the ESTIMATE algorithm. Orange and purple represent a positive and negative correlation, respectively.
References
1. Knighton DR, Hunt TK, Scheuenstuhl H, Halliday BJ, Werb Z, Banda MJ. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science 1983;221:1283–1285.


2. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis: correlation in invasive breast carcinoma. N Engl J Med 1991;324:1–8.

3. Ozdemir F, Akdogan R, Aydin F, Reis A, Kavgaci H, Gul S, et al. The effects of VEGF and VEGFR-2 on survival in patients with gastric cancer. J Exp Clin Cancer Res 2006;25:83–88.

4. Carrillo de Santa Pau E, Arias FC, Caso Pelaez E, Munoz Molina GM, Sanchez Hernandez I, Muguruza Trueba I, et al. Prognostic significance of the expression of vascular endothelial growth factors A, B, C, and D and their receptors R1, R2, and R3 in patients with nonsmall cell lung cancer. Cancer 2009;115:1701–1712.


5. Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res 1995;55:3964–3968.

6. Gunningham SP, Currie MJ, Han C, Robinson BA, Scott PA, Harris AL, et al. VEGF-B expression in human primary breast cancers is associated with lymph node metastasis but not angiogenesis. J Pathol 2001;193:325–332.


7. Neuchrist C, Erovic BM, Handisurya A, Fischer MB, Steiner GE, Hollemann D, et al. Vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 expression in squamous cell carcinomas of the head and neck. Head Neck 2003;25:464–474.


8. Juttner S, Wissmann C, Jons T, Vieth M, Hertel J, Gretschel S, et al. Vascular endothelial growth factor-D and its receptor VEGFR-3: two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol 2006;24:228–240.


9. Chen CN, Hsieh FJ, Cheng YM, Cheng WF, Su YN, Chang KJ, et al. The significance of placenta growth factor in angiogenesis and clinical outcome of human gastric cancer. Cancer Lett 2004;213:73–82.


10. Bhandari V, Hoey C, Liu LY, Lalonde E, Ray J, Livingstone J, et al. Molecular landmarks of tumor hypoxia across cancer types. Nat Genet 2019;51:308–318.



11. Vitale I, Shema E, Loi S, Galluzzi L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat Med 2021;27:212–224.



12. Yoshihara K, Shahmoradgoli M, Martinez E, Vegesna R, Kim H, Torres-Garcia W, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun 2013;4:2612.



13. Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature 2009;462:108–112.


14. Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl) 2015;3:83–92.



15. Steven A, Seliger B. The role of immune escape and immune cell infiltration in breast cancer. Breast Care (Basel) 2018;13:16–21.




16. Srabovic N, Mujagic Z, Mujanovic-Mustedanagic J, Softic A, Muminovic Z, Rifatbegovic A, et al. Vascular endothelial growth factor receptor-1 expression in breast cancer and its correlation to vascular endothelial growth factor a. Int J Breast Cancer 2013;2013:746749.




17. Zang J, Li C, Zhao LN, Shi M, Zhou YC, Wang JH, et al. Prognostic value of vascular endothelial growth factor in patients with head and neck cancer: a meta-analysis. Head Neck 2013;35:1507–1514.


18. Poon RT, Lau C, Pang R, Ng KK, Yuen J, Fan ST. High serum vascular endothelial growth factor levels predict poor prognosis after radiofrequency ablation of hepatocellular carcinoma: importance of tumor biomarker in ablative therapies. Ann Surg Oncol 2007;14:1835–1845.



19. Rubenstein JL, Kim J, Ozawa T, Zhang M, Westphal M, Deen DF, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia 2000;2:306–314.



20. Li J, Han T. Comprehensive analysis of the oncogenic roles of vascular endothelial growth factors and their receptors in stomach adenocarcinoma. Heliyon 2023;9:e17687.



21. Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 1992;359:845–848.



22. Meng Q, Duan P, Li L, Miao Y. Expression of placenta growth factor is associated with unfavorable prognosis of advanced-stage serous ovarian cancer. Tohoku J Exp Med 2018;244:291–296.


23. Guo X, Chen M, Cao L, Hu Y, Li X, Zhang Q, et al. Cancer-associated fibroblasts promote migration and invasion of non-small cell lung cancer cells via miR-101-3p mediated VEGFA secretion and AKT/eNOS pathway. Front Cell Dev Biol 2021;9:764151.



24. Liu Z, Wang J, Mao Y, Zou B, Fan X. MicroRNA-101 suppresses migration and invasion via targeting vascular endothelial growth factor-C in hepatocellular carcinoma cells. Oncol Lett 2016;11:433–438.


25. Liu X, Kwong A, Sihoe A, Chu KM. Plasma miR-940 may serve as a novel biomarker for gastric cancer. Tumour Biol 2016;37:3589–3597.



26. Yang HW, Liu GH, Liu YQ, Zhao HC, Yang Z, Zhao CL, et al. Over-expression of microRNA-940 promotes cell proliferation by targeting GSK3beta and sFRP1 in human pancreatic carcinoma. Biomed Pharmacother 2016;83:593–601.

27. Chen X, Ying X, Wang X, Wu X, Zhu Q, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer deliver microRNA-940 to induce macrophage M2 polarization. Oncol Rep 2017;38:522–528.


28. Chen G, Wang Q, Wang K. MicroRNA-218-5p affects lung adenocarcinoma progression through targeting endoplasmic reticulum oxidoreductase 1 alpha. Bioengineered 2022;13:10061–10070.



29. White J, Jiang Z, Diamond M, Saed G. Hypoxia induces transforming growth factor beta 1 (TGFβ1) in human macrophages through a hypoxia inducible factor 1α (HIF-1α) - dependent mechanism. Fertil Steril 2009;92(3 Suppl):S124.

- TOOLS
- Related articles in GNI








